Research - (2021) Volume 9, Issue 3
Effectiveness of Biodentine in Combination with Diode Laser in Treatment of Dentine Hypersensitivity
*Correspondence: Ibtehal Atia Habeeb, Department of Periodontics, College of Dentistry, University of Baghdad, Iraq, Email:
Abstract
Background: Dentine hypersensitivity is a relatively common problem experienced in clinical dental practice. Aim: To evaluate the efficacy of diode laser alone in comparison with Biodentine +diode laser in the management of dentinal hypersensitivity. Material and methods: A total of 20 patients (90 teeth) with a clinical diagnosis of dentin hypersensitivity were included in this clinical trial. Dentin sensitivity was assessed in response to an air blast stimulus using Schiff cold air sensitivity scale before treatment, 15 min, 2 weeks, and 1 month after the first application. The teeth were randomly divided into two groups. In the first group was treated with diode laser (45 teeth), the second one was treated with Biodentine combination with diode laser (45 teeth). Results: Subjects had treated with Biodentine irradiated with diode laser showed statistically lower (p < 0.05) dentine hypersensitivity compared with the control group (diode laser) immediately after treatment and in follow up. The test group demonstrated a significant reduction in dentine hypersensitivity after 30 min of treatment 2 weeks and one month after the treatment comparing to baseline (0.51 versus 0.80, P= 0.00, 0.18 versus 0.73 P= 0.00 and 0.18 versus 0.71 P=0.00 respectively). Conclusion: The study concluded that both treatment modalities caused significant reduction of dentine hypersensitivity. The combined use of Biodentine and diode laser (976 nm) is the more effective than that of the diode laser (976 nm) alone monitoring dentine hypersensitivity by Schiff scale.
Keywords
DH, Biodentine
Introduction
Dentin hypersensitivity (DH) is a prevalent and painful condition for the patients. The recent definition from the Canadian Advisory Board on dentin hypersensitivity describes DH ËPain derived from exposed dentin in response to chemical, thermal, tactile or osmotic stimuli which cannot be explained as arising from any other dental defect or diseaseË [1]. Recently, the prevalence of DH according to Zeola et al [2], in a systematic review and meta-analysis published in 2019 is between 11% and 33%. It is communally affected individuals at their fourth and fifth span of life [3], causing complement to the patient during eating or even breathing. Mechanism of DH has been explaining by several theories with the hydrodynamic theory suggested by Brännström being the most acceptable one [4]. Rendering to this theory, external stimuli (thermal, mechanical, evaporative and osmotic) causing movement of fluid within the dentinal tubules and this movement secondarily stimulates the pulp nerve ends, producing a painful sensation. So, for DH to arise, there must be two conditions: dentin must be exposed, either by the loss of enamel (attrition, abrasion, erosion, abfraction) or by a gingival recession “lesion localization” and the dentinal tubules must be permeable both to the oral cavity and to the pulp “lesion initiation” [5]. DH management is depending on correct diagnosis, severity localized or generalized condition, prevention or elimination of the causes. These include using jets of air from a triple syringe as thermal stimuli or an exploratory probe as a mechanical stimulus on the exposed surface to aggravate a response from the patient. The severity of pain can be quantifying by using a descriptive scale (slight, moderate, intense) pain or a visual analogue scale (VAS) from 0-10 [6]. Treatment option either invasive or non-invasive in nature. Noninvasive treatment options include dentifrices that contain active ingredient or topical agent Invasive procedures may include gingival surgery, application of resins, or a pulpectomy [7]. For sealing communications between the root and the periodontium Calcium silicate cements are used. Mineral trioxide aggregate (MTA) was suggested for endodontic procedures because it is biocompatible, non-carcinogenic, insoluble in tissue fluids and dimensionally stable. In contrast for earlier material MTA have a higher prevalence of dentin bridge formation. MTA also generates a biocompatible condition in periodontal tissues and when used in perforation area can stimulate cement genesis [8]. To defeat the drawbacks of MTA, Biodentine® (Septodont, Saint-Maur-des Fossés, France) was presented. In a case account in 2012 by Firla [9], mineralization was induced after application of Biodentine. The alkaline effect of Biodentine in the setting reaction make the micromechanical adhesion. The dentin substitute mass can enter the exposed dentin canaliculi due to the alkaline environment at the boundary area of contact between Biodentine and hard tooth surface. Prior studies have proposed that Biodentine is bioactive since it rises proliferation of the pulp cell line OD21 and that it can be reflected as a suitable material for clinical signals of dentinepulp complex regeneration [10]. Lasers, be able to occlude dentinal tubules partially or totally due to their capability to melt peritubular dentin, and consequently reduce hypersensitivity symptoms [11]. Previous study estimating the efficacy of diode lasers (810 and 980 nm) on dentine surface at different parameters showed that these lasers used at 0.8 and 1 W for 10 seconds in continuous mode were capable to seal the dentin tubules. These parameters may be considering effective in the treatment of dentinal hypersensitivity and at the same time can be considered safe for pulp vitality [12]. The use of a diode laser, with a wavelength among 655 and 980 nm, can quicken wound healing by the assistance of collagen synthesis, augmentation of growth factor release and elevation of angiogenesis. Also, vitro studies suggested bactericidal and detoxification effect of diode lasers [13) and can avoid ablation of the root surface, consequently decreases the risk of denuded normal root tissue [14]. This study was carried out to evaluate the efficacy of Biodentine in combination with diode laser in the management of dentinal hypersensitivity.
Materials and Methods
This clinical trial started with detailed medical and dental history from every patient. Twenty patient (25-50) years complaining of dentinal hypersensitivity in 90 teeth due to non caries cervical lesion (NCCL) which include erosion, abrasion and abfraction were enrolled in the study. Exclusion criteria included: carious lesions, defective restorations, enamel cracks, active periodontal disease, periodontal surgery (last 6 months), reversible pulpitis, analgesic or anti- inflammatory treatment (last 72 h), bleaching procedure (last 3 months), desensitizing products (last 6 weeks), pregnant and lactating women. This study approved by ethical committee/ college of Dentistry/ University of Baghdad, follow the guidelines of Helsinki and Tokyo for humans (the reference no. 130619 in 2\12\2019) and an informed written consent was obtained. The trial took place at the summer dental specials center. Previous to their first visit, patients received oral hygiene instructions. At first visit adjacent teeth were isolated by cotton rolls and operator finger. For evaluating the level of pain before any treatment for each tooth, two type of stimuli were used: a tactile stimulus by passing the tip of exploration probe along the cervical area until the patient felt pain. Second stimuli(thermal-evaporative) was done after 3 min which include using jet of air from the same dental equipment was applied for 3 s at a distance of 2 mm and perpendicularly to the tooth cervical area by using Schiff cold air sensitivity scale [15] and as followed:
0.Tooth/subject did not respond to the air stimulus
1. Tooth/subject responded to the air stimulus but did not request discontinuation of the stimulus.
2. Tooth/subject responded to the air stimulus and requested discontinuation or moved from the stimulus.
3. Tooth/subject responded to the air stimulus, considered the stimulus to be painful, and requested discontinuation of the stimulus.
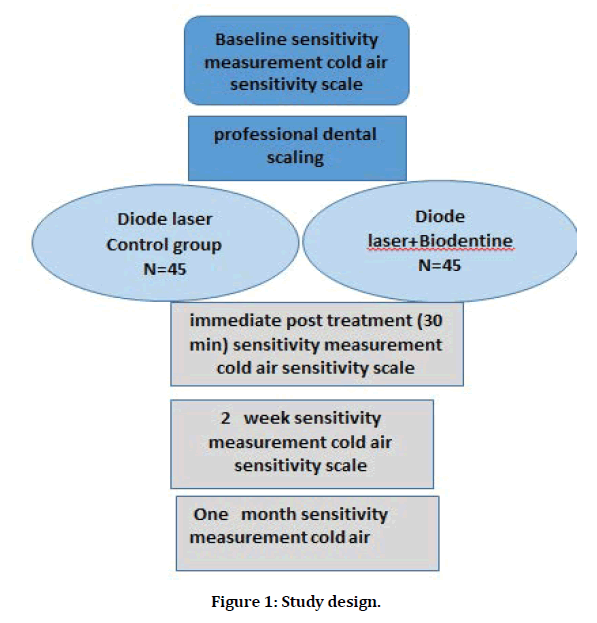
The study design was shown in Figure 1. In this split-mouth study, the patients did not know what kind of therapy each tooth was receiving. The teeth of the different quadrants received different desensitizers, and adjacent teeth received the same treatment. sensitive teeth were randomly divided in to two groups:
Figure 1: Study design.
Group1 (45 teeth) treated with the diode laser (solase, lazon medical laser company) as control group with continuous emission (nm) on non-contact mode (0.5 mm from the surface). The laser device was used with the following parameters: output power of 0.6 mw, irradiation time of 60 secs, 400 tip diameter.
Group2 (45 teeth) treated using both Biodentine and diode laser. Biodentine using according to manufacture instruction, the material is prepared by adding 5 drops of liquid to powder present in the capsule. These component were then triturated with an amalgamator for 30 s at 4000 rpm for the formation of paste of creamy consistency [16]. Biodentine left on tooth surface about 60 s before irradiation with previous diameter in group1, by this way, the laser system might enhance the permanency of Biodentine for a longer time than when it uses alone. DH after treatments was assessed with Schiff scale :30 min after treatment, at 2 weeks, and 1 months after treatments. All patients were instructed to use a soft toothbrush and a toothpaste without any anti-hypersensitivity agent.
Statistical analysis
The following methods of statistical analysis were used in this study. The data were entered in Microsoft Excel and statistical analysis were performed using the Statistical Package for Social Science (SPSS ver 25) software. Independent sample –t-test used to test the difference between two groups.
Results
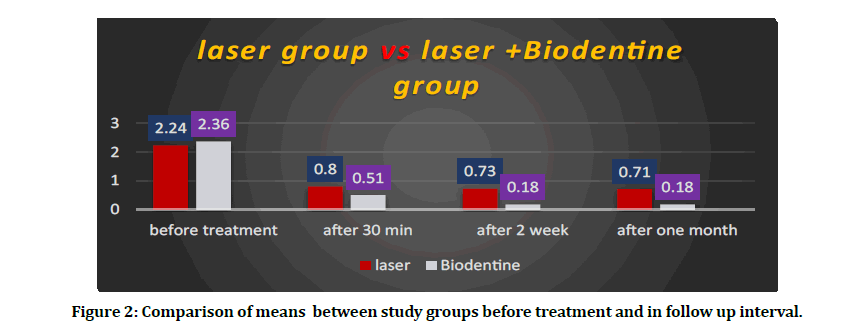
Table 1 showed the percentage distribution of teeth according to score in Schiff scale in both study groups. From this Table 1 in Biodentine +diode laser group we can observe that the highest percentage of scale 2 and 3 was 37.7 ,48.8 respectively before treatment on the other hand in follow up interval after one month we observed that higher percentage of scale 1 and 0 was 17.7, 82.2 respectively. In the Table 2 showed the reduction of mean in diode laser group from 2.24 before treatment to 0.71 after one month however in biodentine +diode laser group the reduction of mean was 2.36 before treatment to 0.18 after one month. Figure 2 showed the comparison of means between study groups before treatment and in follow up intervals.
Table 1: The percentage distribution of teeth according to score in Schiff scale in studies groups.
| Degree of DH Schiff cold air sensitivity scale | Before treatment Immediately | Immediately 30 min after the first application | 15 days after the first application | 30 days after the first application | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diode laser group | Diode+ Biodentine | Diode laser group | Diode+Biodentine group | Diode laser group | Diode+Biodentine group | Diode laser group | Diode+Biodentine group | |||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | |
| 0 | 0 | 0 | 0 | 0 | 9 | 20 | 22 | 48.8 | 12 | 26.6 | 37 | 82.2 | 12 | 26.6 | 37 | 82.2 |
| 1 | 7 | 15.5 | 6 | 13.3 | 36 | 80 | 23 | 51.1 | 33 | 73.3 | 8 | 17.7 | 33 | 73.3 | 8 | 17.7 |
| 2 | 20 | 44.4 | 17 | 37.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | 18 | 40 | 22 | 48.8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Table 2: Comparison between studies groups using independent sample t- test.
| GROUP/Time | laser | Laser +Biodentie | Independent sample t-test | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 95% confidence interval | |||||||||
| Mean | SD | Mean | SD | lower | upper | t-test | Df | P value | |
| Before treatment | 2.24 | 0.71 | 2.36 | 0.71 | 0.4 | 0.18 | 0.74 | 88 | 0.72 |
| After 30 minute | 0.8 | 0.4 | 0.51 | 0.5 | 0.09 | 0.48 | 2.99 | 88 | 0 |
| After 2 week | 0.73 | 0.44 | 0.18 | 0.38 | 0.38 | 0.73 | 6.73 | 88 | 0 |
| After one month | 0.71 | 0.45 | 0.18 | 0.38 | 0.35 | 0.71 | 5.96 | 88 | 0 |
Figure 2: Comparison of means between study groups before treatment and in follow up interval.
Discussion
Dentin hypersensitivity (DH) is a localized, short and sharp pain in response to thermal, mechanical chemical, or osmotic stimuli, finishing after removal of the stimulus. The etiology is many and the factors involved are vague [17]. In this clinical trial we compared the efficacy of Biodentine and diode laser with diode laser alone for reduction of dentine hypersensitivity. Mineral trioxide total (MTA) is a bioactive material made out of Portland concrete and bismuth oxide. Numerous examinations have demonstrated that MTA can instigate hard tissue formation, and past investigations have indicated the impact of MTA on cementoblasts and odontoblast [18]. MTA likewise makes a biocompatible situation in periodontal tissues and when used in perforating area stimulate cementogenesis. Past examinations have analyzed the impacts of MTA in vitro on the expansion of oral keratinocytes and cementoblasts, and thought about White MTA (WMTA) with dark MTA (GMTA). It was discovered that compared with cementoblasts grown on GMTA. It was found that cementoblast proliferation expressively increased when grown on the surface of W MTA [19].
Biodentine another tricalcium silicate (Ca3SiO5) could be both temporary restoration and final dentine standby. Its high compression strengths, perfect sealing properties, and little setting time [20]. When Compared to MTA, Biodentine is simpler to deal with [21]. It tends to be utilized as a temporary filling as long as a half year and in various applications as permanent dentin substitute with no surface treatment. Also, while discoloration with MTA [22] and its byproducts have been stated in regenerative endodontics and appear to be mostly due to Bismuth oxide as a radio-opacifier [23], no change in color of tooth crown has been stated after 4 years with Biodentine due to presence of Zirconium oxide as a radio-opacities [24].
Biodentine prompted tertiary dentin synthesis when useful as indirect or direct pulp capping material in rodent teeth [25]. Connections of Biodentine with the dentin gave prompts to seeing how this material gives a minimal fixing with no dentin surface inclination: no bonding no etching. In a test work, dentin cuts were arranged and Biodentine was prepared and blended in with a fluorescent color before its application onto the dentin surface. scanning electron microscopy and Confocal laser scanning electron microscopy checking interface between Biodentine and dentin. Confocal laser checking electron microscopy discovered that Biodentine infiltrated into the dentin tubules framing tag-like structures into the dentin tubules. Scanning electron microscopy discovered that the dentin tubules seemed with plugs of mineralization crystals just under the border obliterating the dentin tubules. These outcomes clarify the marginal close up on side and the micromechanical retention of the material on another side [26].
The results of the present clinical trial demonstrated that both therapies resulted in overall relief in dentine hypersensitivity. However, the Biodentine +diode laser group was superior compared with diode laser group.
Lasers, through their capacity to soften peritubular dentin, can somewhat or fully occluded dentinal tubules, and in this manner lessen patients' symptoms. Numerous examinations have been done centering on the efficiency of the utilization of diode lasers for dentinal hypersensitivity. Matsumoto et al. indicated 85% improvement in teeth treated with laser. Yamaguchi et al. saw a successful enhancement index e of 60% in the group treated with laser matched with 22.2% in the control non-lased group. In another examination results indicated an improvement of 69.2% in the group treated with laser contrasted with 20% in the placebo treatment group [27].
In this clinical trial study we using solase diode laser with wavelength 976 with an optical fiber (400 mm diameter),0.6 w in non-contact mod for 60 seconds. Several investigations have stated that the use of 980nm diode laser could be utilized safely in endodontic treatment and in root canal disinfection [28].
Conversely, limited studies have stated on the interaction of 980nm diode laser energy with the dentin surface consequence auxiliary changes.
More investigations are required to decide if the 980nm diode laser can treat dentine sensitivity successfully, like another type of lasers [29]. However, in this study using 5 J/cm2 energy density diode laser we observed reduction in the level of DH to thermal-evaporative stimuli using Schiff scale show a reduction of the mean from 2.24 before treatment to 0.8 after treatment and these results remain stable up to one month, in agreement with most studies using the same parameter. Fabio et al in 2016 [30] show results of good dentinal tubule occlusion when specimens were irradiated by using of a galium: aluminum: arsenide (GaAlAs) diode laser used was a 970nm wavelength laser with an optical fiber (200 mm diameter). The specimens were irradiated with a frequency of 10 Hzincontactmode for 30 seconds. In a comparable report utilizing a diode laser in continuous, o noncontact modes (1 mm) conveyed power densities every second 2547, 3184, 5092, and 6366J/cm2 with power setting: 0.8, 1, 1.6, and 2 W. The optical fiber width was 200 μm. and irradiant seed was 1mm Illumination speed in the examination was 1mm/sec [31].
A few investigations have showed a synergistic activity of lasers in adjunctive with desensitizing material. These examinations exhibited that lasers can improve the permanence of the desensitizer for longer time than when they are used alone Consequently, if a laser device is utilized in adding to a traditional desensitizing agent, the last stays over the tooth surface for 60 seconds before the irradiation [32].
However, Biodentine® and diode laser were used in combination on the tooth surface where the dentinal surface was exposed in contact mode at 5 J â??cm² joules for 1 minute on each tooth. The results of this clinical study shows a reduction of dentine hypersensitivity by mean of 2.36 before treatment to 0.51 after 30 minutes and these results agree with Vitro study of Sameera et al 2019 [33]., so that it can be used in treating dentinal hypersensitivity. Lasers through their ability to melt peritubular dentin, can partially or totally occlude dentinal tubules, and therefore reduce patients’ symptoms of hypersensitivity.
Conclusion
Within the limitations of this study it was concluded that ® Bio-dentine® in combination with diode laser is effective in reduction of dentine hypersensitivity compared with diode laser alone, it may be due to effect of diode laser on surface of tooth that may enhance penetration of Biodentine in dentinal tubules.
References
- https://www.cda-adc.ca/jcda/vol-69/issue-4/221.pdf
- Zeola LF, Soares PV, Cunha-Cruz J. Prevalence of dentin hypersensitivity: Systematic review and meta-analysis. J Dent 2019; 81:1-6.
- Rees JS, Jin LJ, Lam S, et al. The prevalence of dentine hypersensitivity in a hospital clinic population in Hong Kong. J Dent2003; 31:453-461.
- Brannstrom M. A hydrodynamic mechanism in the transmission of pain-producing stimuli through the dentine. Sensory Mechanisms Dentine 1963; 73-79.
- Addy M. Dentine hypersensitivity: New perspectives on an old problem. Int Dent J 2002; 52:367-375.
- Gillam DG, Orchardson R. Advances in the treatment of root dentine sensitivity: mechanisms and treatment principles. Endodont Topics 2006; 13:13-33.
- Dilsiz A, Aydın T, Emrem G. Effects of the combined desensitizing dentifrice and diode laser therapy in the treatment of desensitization of teeth with gingival recession. Photomedicine Laser Surg 2010; 28:S69.
- Toptanci IR, Dalli M, Colak H. The composition and biologic action of mineral trioxide aggregate: A review. Konuralp Med J 2013; 5:70-74.
- Firla MT. Direct pulp capping with a bioactive dentine substitute. Oral Health. 2012; 102:40.
- Zanini M, Sautier JM, Berdal A, et al. Biodentine induces immortalized murine pulp cell differentiation into odontoblast-like cells and stimulates biomineralization. J Endodont 2012; 38:1220-1226.
- Gholami GA, Fekrazad R, Esmaiel-Nejad A, et al. An evaluation of the occluding effects of Er; Cr: YSGG, Nd: YAG, CO2 and diode lasers on dentinal tubules: A scanning electron microscope in vitro study. Photomedicine Laser Surg 2011; 29:115-121.
- Umana M, Heysselaer D, Tielemans M, et al. Dentinal tubules sealing by means of diode lasers (810 and 980 nm): A preliminary in vitro study. Photomedicine Laser Surg 2013; 31:307-314.
- Harris DM, Yessik M. Therapeutic ratio quantifies laser antisepsis: Ablation of porphyromonas gingivalis with dental lasers. Lasers Surg Med 2004; 35:206-213.
- Caruso U, Nastri L, Piccolomini R, et al. Use of diode laser 980 nm as adjunctive therapy in the treatment of chronic periodontitis. A randomized controlled clinical trial. New Microbiol 2008; 31:513-518.
- Schiff T, Delgado EV, Zhang YP, et al. Clinical evaluation of the efficacy of an in-office desensitizing paste containing 8% arginine and calcium carbonate in providing instant and lasting relief of dentin hypersensitivity. Am J Dent 2009; 22:8A-15A.
- Laurent P, Camps J, De Méo M, et al. Induction of specific cell responses to a Ca3SiO5-based posterior restorative material. Dent Materials 2008; 24:1486-1494.
- Absi EG, Addy M, Adams D. Dentine hypersensitivity: A study of the patency of dentinal tubules in sensitive and nonâ?sensitive cervical dentine. J Clin Periodontol 1987; 14:280-284.
- Paranjpe A, Zhang H, Johnson JD. Effects of mineral trioxide aggregate on human dental pulp cells after pulp-capping procedures. J Endodont 2010; 36:1042-1047.
- Oviir T, Pagoria D, Ibarra G, Geurtsen W. Effects of gray and white mineral trioxide aggregate on the proliferation of oral keratinocytes and cementoblasts. J Endodont 2006; 32:210-213.
- Villat C, Tran VX, Pradelle-Plasse N, et al. Impedance methodology: A new way to characterize the setting reaction of dental cements. Dent Materials 2010; 26:1127-1132.
- Koubi G, Colon P, Franquin JC, et al. Clinical evaluation of the performance and safety of a new dentine substitute, Biodentine, in the restoration of posterior teeth—A prospective study. Clin Oral Investigations 2013; 17:243-249.
- Parirokh M, Torabinejad M. Mineral trioxide aggregate: A comprehensive literature review—part III: Clinical applications, drawbacks, and mechanism of action. J Endodont 2010; 36:400-413.
- Vallés M, Mercadé M, Duran-Sindreu F, et al. Influence of light and oxygen on the color stability of five calcium silicate–based materials. J Endodont 2013; 39:525-528.
- Marconyak LJ, Kirkpatrick TC, Roberts HW, et al. A comparison of coronal tooth discoloration elicited by various endodontic reparative materials. J Endodont 2016; 42:470-473.
- Tran XV, Gorin C, Willig C, et al. Effect of a calcium-silicate-based restorative cement on pulp repair. Journal of dental research. 2012; 91:1166-1171.
- Atmeh AR, Chong EZ, Richard G, et al. Dentin-cement interfacial interaction: calcium silicates and polyalkenoates. J Dent Res 2012; 91:454-459.
- Kumazaki M, Zennyu K, Inoue M, et al. Clinical evaluation of GaAlAs-semiconductor laser in the treatment of hypersensitive dentin. Japanese J Conservative Dent 1990; 33:911-918.
- Faria MI, Sousa-Neto MD, Souza-Gabriel AE, et al. Effects of 980-nm diode laser on the ultrastructure and fracture resistance of dentine. Lasers Med Sci 2013; 28:275-280.
- Liu Y, Gao J, Gao Y, et al. In vitro study of dentin hypersensitivity treated by 980-nm diode laser. J Lasers Med Sci 2013; 4:111.
- Rizzante FA, Maenosono RM, Duarte MA, et al. In Vitro evaluation of dentin hydraulic conductance after 980 nm diode laser irradiation. J Periodont 2016; 87:320-326.
- Umana M, Heysselaer D, Tielemans M, et al. Dentinal tubules sealing by means of diode lasers (810 and 980 nm): A preliminary in vitro study. Photomed Laser Surg 2013; 31:307-314.
- Kumar NG, Mehta DS. Shortâ?term assessment of the Nd: YAG laser with and without sodium fluoride varnish in the treatment of dentin hypersensitivity–A clinical and scanning electron microscopy study. J Periodont 2005; 76:1140-1147.
- Sameera U, Bilichodmath S, Paul P. Evaluation of the efficacy of strontium chloride, biodentine® and biodentine® in combination with diode laser in the management of dentinal hypersensitivity-An in vitro SEM Study. J Int Academy Periodont 2019; 22:74-81.
Author Info
Department of Periodontics, College of Dentistry, University of Baghdad, IraqCitation: Ibtehal Atia Habeeb, Maha SH Mahmood, Effectiveness of Biodentine in Combination with Diode Laser in Treatment of Dentine Hypersensitivity, J Res Med Dent Sci, 2021, 9 (3):103-109.
Received: 16-Feb-2021 Accepted: 11-Mar-2021 Published: 18-Mar-2021