Review Article - (2022) Volume 10, Issue 11
âEffect of Tocilizumab in Reducing Mortality and Need for Invasive Ventilation in a COVID-19 Infected Patientsâ
Sonali Pathak* and Sarika Dakhode
*Correspondence: Sonali Pathak, Department of Community Medicine, Jawaharlal Nehru Medical College, Datta Meghe Institute of Medical Sciences, Sawangi (Meghe), Wardha, Maharashtra, India, Email:
Abstract
Coronavirus disease 2019 has claimed the lives of almost 2.5 million people, yet no viable therapy or care has yet been established. To learn more about Tocilizumab treatment for COVID-19 patients, studies were conducted.
The 2019 Coronavirus disease outbreak has created havoc and posed a significant epidemiological, diagnostic, and therapeutic challenge. The availability of vaccines has reduced the number of serious cases daily, but it hasn't reduced the need for appropriate treatment. The second wave hit the country in a big way and claimed lives in big numbers. Researchers are still looking for a viable treatment using medications that have been shown to help with other diseases. From the clinician’s side treatment angles have changed as time has elapsed. The experience has grown and we have a much better understanding of the disease in to remdesivir, low molecular weight Heparin, and Dexamethasone were the key recommendations initially. However, because the pathophysiology of COVID-19 is complex, treatment should involve suppression of the pro inflammatory cytokine in addition to antiviral action and coagulopathy treatment.
Tocilizumab, a recombinant anti IL6 receptor monoclonal antibody, has been approved for use for cytokine release syndrome due to COVID-19.
Recently, an antibody cocktail drug which is a combination of Casirivimab and Imdevimab has got approval by FDA and is available in the market now which has shown positive outcomes in infected patients. Another drug Sotrivimab which is a new COVID monoclonal antibody has been released by FDA as emergency use authorization.
Keywords
COVID-19, Tocilizumab, Ventilation, Mortality
Introduction
The Coronavirus disease 2019 outbreak has spread very fast across the globe, resulting in a global pandemic situation. By April 29, 2021, the World Health Organization (WHO) had received over 149,000,000 confirmed COVID-19 cases, with over 3100000 deaths. Due to its great pestilence, this unique Corona virus dis also known as Coronavirus disease 2019 (COVID-19), has spread very rapidly all over the world. Virus has overwhelmingly veiled ‘SARS’ and ‘MERS’ in terms of number of sick people and the geographic purview of epidemic regions. The on-going COVID-19 outbreak has assumed an altitude of a serious danger to people across the globe.
New strains of the virus have emerged, posing a potential danger because infections have been observed in people who have been fully vaccinated.
Viruses become variants when their genes are altered or mutated. The
• ‘Delta’ variant and
• Omicron
Variant are two prominent variants of contemporary concern.
‘Delta’ variant: It's more contagious in the ‘Delta’ version. The ‘Delta’ variant has substantially more infectiousness than early variants, with a transmissibility of more than twice that of earlier variants. In two different trials from Canada and Scotland, patients infected with the ‘Delta’ variant were more likely to be hospitalised than patients infected with the original virus that causes COVID-19. Despite this, the great majority of COVID-19 related hospitalizations and deaths are caused by unvaccinated patients [1]. Unvaccinated people are still a major source of concern: They pose the greatest risk of transmission since they are more likely to become infected and disseminate the virus. COVID-19 (also known as breakthrough infections) is less likely in those who have been fully vaccinated than in those who have not been. People, who are infected with the ‘Delta’ version of the virus, including those who have received full vaccination but have had clinical breakthrough infections, can disseminate it to others.
Omicron variant: On November 26, 2021, WHO recognized the mutant B.1.1.529 as a variant of concern, called Omicron, on the recommendation of WHO's technical advisory Group on virus evolution? Based on details supplied to the TAG-VE, it was determined that Omicron has a variety of mutations that could change how it behaves, such as how deftly it propagates or the severity it produces [2].
Transmissibility: It's uncertain whether Omicron is more transmissible (easier to spread) than other variants like Delta. The number of persons testing positive has grown in areas of South Africa affected by this variant, although epidemiologic studies are being planned to ascertain if this is attributable to Omicron or other reasons [2].
Prior SARS-CoV-2 infection's effectiveness: Preliminary research suggests that Omicron may have a higher probability of reinfection than other variations of concern (that is those who were infected previously may be more easily reinfected with Omicron), although data is limited. In the next days and weeks, more information about this will become accessible [2].
Vaccine effectiveness: WHO is collaborating with technical partners to assess the impact of the change on current counter measures, such as vaccinations? Vaccination, particularly against the most common type, 'Delta,' is critical in reducing severe illness and mortality. Immunizations that are now available are still effective in avoiding serious disease and death [2].
Current treatments efficacy: Corticosteroids and IL6 receptor blockers Tocilizumab will continue to be a success in the treatment of severe COVID-19 disease. Other treatments will be evaluated to see whether they are still effective in light of the Omicron variant's changes to the virus [2].
Literature Review
Pathophysiology of SARS-CoV-2: The first major step whether it be in upper respiratory tract or lower respiratory tract the virus attaches on to receptors in lungs with the help of spike proteins [3]. Spike proteins have 2 subunits S1 and S2.
Main subunit is S1 as it’s the reason for maximum infection occurring in lungs. They have a receptor binding domain and combines with ACE2 receptor on type 2 alveolar epithelial cells. S2 subunit helps in fusion of virus to the host cell membrane.
There are so many receptors which the virus binds to but the most important of them is angiotensin converting enzyme receptor. It is the single most important step. There is a co receptor called TMPR SS2.
After this virus enters type 2 alveolar epithelial cells. Viral RNA is released and is going to recruit many endoplasmic reticulum and golgi bodies because they are required for synthesis of many copies of RNA and structural proteins [3].
Assembling and formation of new virus particle takes place and are released from alveolar epithelial cells with the help of budding [3].
In severe COVID infection, direct damage is caused to type 2 alveolar epithelial cells by apoptosis which leads to loss of surfactant so that friction capacity is gone. The virus also recruits neutrophil and macrophages which releases a large number of inflammatory mediatorscytokines which released in large amounts leads to cytokine storm [3].
As a result there is more inflammation, multi organ damage, multisystem failure and multi organ failure [3].
Because of the damage, there will be leaky capillaries in the alveoli, alveolar oedema and there will be features of ARDS. ARDS will be having diffuse alveolar damage as a result of which the alveoli will be covered by hyaline membrane. As a result there will be no exchange of gases [3].
Transmission
Respiratory droplets: By sneezing and coughing. They persist in the environment for 8-15 minutes.
Aerosol: Persist in the environment for 3 hours.
Direct contact: Hand to face most dreadful thing and is major step for prophylaxis and prevention. Frequent hand wash for 30 seconds is advised.
Fomite: On woods and glasses for 5 days, plastics and steel 3 days, aluminium and copper for 8 hours [3].
Incubation period: Time period between exposure to symptom onset=2 to 14 days; median=5th day. This is why quarantine is given for 14 days from the last exposure to confirmed case. It is very important to curb the infection in community [3].
Another step to prevent the spread is social distancing of 1 meter that is 3 feet as per WHO and 1.8 meter that is 6 feet as per CDC [3].
Clinical features: It has a classical triad of fever, cough and dyspnoea in almost 15% of the patients [4].
Other features: Tiredness, headache, nausea, vomiting, abdominal pain [4]. Earliest symptom is loss of smell and taste. In children, multisystem inflammatory syndrome resembles to Kawasaki disease and toxic shock syndrome.
One of the fearsome complications is hypercoagulability which leads to increased risk of thrombosis. Platelet count increased, prothrombin time is increased, FDP is increased, and D-dimer is increased and associated with increased risk of death. Leads to complications like deep vein thrombosis and pulmonary embolism [4].
Clinical spectrum
• Mild COVID-19:
• Respiratory rate: less than 24 per minute
• Percentage saturation of oxygen: More than 94
• Moderate COVID-19:
• Mild to moderate symptoms of pneumonia
• Respiratory rate 24-30 per minute
• Percentage saturation of oxygen: 90-94
• Severe COVID-19:
• Severe symptoms of pneumonia
• Respiratory rate more than 30 per minute
• Percentage saturation of oxygen: Less than 90
• Septic shock
• ARDS
Terminologies
Suspect case: 2 or more of the following symptoms appear suddenly: Headache, myalgia, throat discomfort, runny nose, shortness of breath, lack of appetite, nausea and vomiting, diarrhoea, changed mental condition.
Probable case: A suspect patient who is a confirmed case's contact or is epidemiologically connected to a cluster of confirmed cases OR asymptomatic individual who is a high risk contact of a confirmed case or is epidemiologically connected to a confirmed case cluster OR.
If a physician suspects it clinically, OR individuals experiencing respiratory distress before to death who were a contact probable or confirmed case or epidemiologically connected to a cluster of confirmed cases, death not otherwise defined.
Confirmed case by lab: Regardless of clinical indications and symptoms, a person with lab confirmation.
• Lab investigations
• WBCS will be decreased
• Low lymphocyte count
• Increased PT and D dimer
• Increased ALT, AST, LDH and CRP [5,6].
• Diagnostic test: rapid antigen test and Reverse transcriptase polymerase chain reaction.
• Chest x ray-most common finding is ground glass opacities in lower lobes.
Lung ultrasound pocus:
• Early detection of sub pleural consolidation; increased focal B lines; late/severe diffuse confluent B lines.
• HRCT chest ground glass opacities and crazy pavement pattern.
Treatment options
• Entry inhibitors: Bamlanivimab, Imdevimab,
• Prevention of uncoating: Hydroxychloroquine
• RNA polymerase inhibitor like Ramdesivir, Favipiravir, Molmipiravir, Lopinavir
• Reduction in cytokine storm: steroids like Methyl Prednisolone and Dexamethasone. Anticoagulants like low molecular weight heparin
• Interleukin-6 inhibitor: Tocilizumab medication has been shown to have therapeutic advantages in COVID-19 patients in recent studies (Figure 1) [5,6].
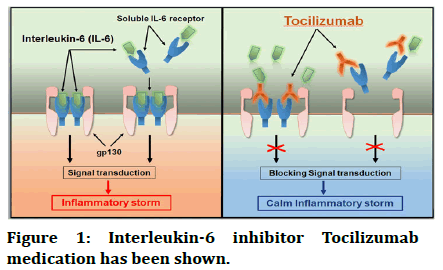
Figure 1: Interleukin-6 inhibitor Tocilizumab medication has been shown.
Tocilizumab dosage: Tocilizumab is a treatment option for Rheumatoid arthritis, Juvenile Idiopathic Arthritis (JIA), and giant cell arteritis.
The FDA has issued an emergency use authorization of Tocilizumab for the treatment of COVID-19 in hospitalized adults and paediatric patients (aged >2 years) who require ventilator support and on high dosage corticosteroids [7].
Dose: 8 mg/kg IV; maximum dose is 800 mg in patients more than 18 years of age.
In patients less than 18 years of age: If more than 30 kg body weight 8 mg/kg; if less than 30 kg body weight 12 ml/kg. Route Is I.V. infusion over 60 minutes. If clinical signs or symptoms increase or do not improve after the initial IV infusion, a second IV infusion may be given 8 hours later [7]. SC administration is not approved for COVID-19 therapy.
Indication
Patient should meet all criteria: Rapidly worsening respiratory status over 24-48 hours despite at least 24 hours of steroid use, temperature more than 38.3 degree celsius for last 24 hours, absolute lymphocyte count of less than 1000 mm3, elevated serum inflammatory markers-LDH more than 500 units/L, ferritin more than 1000 ng/ml or doubling within 24 hours, D dimer more than 5 mg/L, patient does not have a poor prognosis due to another cause, absence of systemic bacterial/ fungal infection.
Risks and side effects: Oral ulcers, coughing and throat problems, blocked or runny nose, headaches or dizziness, hypercholesterolemia (high blood pressure) (increased cholesterol in the blood), aching muscles, shortness of breath, tight chest, wheezing, high temperature, weight gain or swollen ankles, skin rashes, secondary infections, itching.
Contraindication: Patients with increased risk of gastrointestinal perforation, ALT/AST ratio more than 3 times upper limit neutropenia of less than 500 mm3, thrombocytopenia, latent/active pulmonary TB.
Studies showing effect of Tocilizumab on COVID-19 patients. In all, 60% patients showed clinical improvement, 15% patients died, 55% patients were discharged alive, and 30% patients stayed in the hospital. After their clinical condition improved, 36.1% were given concomitant steroids [8]. Total 87% patients were admitted to the critical care unit, with 48.3% requiring invasive mechanical breathing.
Discussion
Only a few patients could undergo a repeat IL6 testing but the results showed markedly increased levels in blood. Tocilizumab's competitive binding to the IL6 receptor induces a short collection of free IL6 in the blood, which is thought to cause the effect [9]. There was also an increase in D dimer, which was highest on day seven and then dropped. LDH and procalcitonin did not reveal any obvious patterns, indicating that they are not COVID-19 specific indicators. The effects of oxygen supported individuals after Tocilizumab administration in COVID-19 patients show mixed results. Oxygen support was observed to improve in some experiments, but not in others [10]. It was observed that there was an overall increase in Ratio of arterial oxygen partial pressure to fractional inspired oxygen after Tocilizumab, although it's unclear whether this was due to the medicine or was more likely due to the natural course of ARDS. In one of the studies [11], Tocilizumab was found to have nil effect on FiO2 decline. Bal most after a month, 44.8% patients needed to be extubated. COVID-19 extubation rates have only been published in a small study after taking Tocilizumab; two out of three patients were successfully extubated.
By the end of the study, 60% had shown improvement and 55% were discharged alive? Somers et al. [12] reported a discharge rate of 56%. We discovered a 15% 30 day death rate, which is within the range (13-27%) reported in previous studies [9]. Many studies have looked into the link between Tocilizumab and COVID-19 patient mortality; however the results have been mixed. According to Salvarani and Campochiaro [12-14], patients who received Tocilizumab had no major difference in mortality. Tocilizumab, on the other hand, has been linked in many trials to a lower risk of death, and all cause mortality. It's also worth noting that several of these trials used steroids concurrently, which is a known confounder for increased survival rates. Despite having the same COVID-19 illness severity at the outset, more patients were dying, had less clinical improvement, and were discharged. It was believed that this is related to the larger steroid doses; most patients got doses of more than 6 mg daily of dexa, making them immune suppressed compared to the recovery group. Another aspect contributing to the poor outcomes when steroid and Tocilizumab were combined was that the steroid group had a greater risk of infectious problems, albeit this was not statistically significant. Tocilizumab has been associated to secondary infections due to its immune suppressive properties. It's also worth mentioning that many of these studies permitted concurrent steroid use, which is a recognised confounder for improved survival. Even while the COVID-19 sickness severity was the same at the start, more patients died, had less clinical progress, and were released alive. We believe this is due to the higher steroid dosages used in our trial; most patients received doses of more than 6 mg daily of Dexamethasone equivalents, which rendered them immune suppressed in comparison to the recovery group. Another factor contributing to the poor results when steroid and Tocilizumab were used together is that the steroid group had a higher rate of infectious issues, however this was statistically insignificant.
Many of these trials also allowed for concomitant steroid usage, which has been demonstrated to improve survival. Despite the fact that the severity of the COVID-19 illness remained the same at the outset, more patients died, had less clinical progress, and were released alive. We believe this is related to the greater steroid dosages utilised in our trial; most patients got more than 6 mg daily of dexamethasone equivalents, making them immunosuppressed when compared to the recovery group. Another aspect contributing to the poor outcomes when steroid and Tocilizumab were combined was that the steroid group had a greater risk of infectious concerns, albeit this was not statistically significant.
Tocilizumab is connected to secondary infections and is immune suppressive [10]. The secondary infections mainly include bacteraemia, hospital acquired pneumonia and ventilator associated pneumonia. Fungal infections are also not less common as per studies.
Conclusion
Tocilizumab treatment did not result in lowering of mortality in patients with severe COVID-19. Due to observational nature of all investigation, these findings will be needed to get confirmed in different clinical studies to evaluate the treatment's effectiveness.
The key role in reducing the infection rate and graveness of the novel virus is vaccination. There are 4 kinds of vaccines available as now: Novavax which is a recombinant protein subunit vaccine, Pfizer BioNTech and moderna which is nucleic acid vaccine mRNA based COVAXIN and sinovac which is whole virus vaccine, COVISHIELD, sputnik, janssen.
People should follow all safety norms despite vaccination in order to break this chain of transmission. Travelling less is of utter importance if not required. Getting an RTPCR report if facing any symptoms even if it is the slightest. Isolating oneself in case symptomatic is a character of a responsible system.
Conflict of Interest
I hereby declare that i have no conflict of interest with the above article.
References
- Centres for Disease Control and Prevention (CDCP). Coronavirus disease 2019 (COVID-19). U.S. Department of Health and Human Services, 2021.
- World Health Organisation (WHO). Update on Omicron. 2021
- Vallamkondu J, John A, Wani W, et al. SARS CoV-2 pathophysiology and assessment of Coronaviruses in CNS diseases with a focus on therapeutic targets. Biochim Biophys Acta Mol Basis Dis 2020; 1866:165889.
- da Rosa Mesquita R, Francelino Silva Junior L, Santos Santana F, et al. Clinical manifestations of COVID-19 in the general population: Systematic review. Wien Klin Wochenschr 2020; 133:377-382.
- Martinez Sanz J, Muriel A, Ron R, et al. Effects of Tocilizumab on mortality in hospitalized patients with COVID.19: A multicentre cohort study. Clin Microbiol Infect 2021; 27:238–243.
- Kewan T, Covut F, Al Jaghbeer MJ, et al. Tocilizumab for treatment of patients with severe COVID-19: A retrospective cohort study. Eclin Med 2020.
- Novel Coronavirus Resource Center (NCRC). Actemra (tocilizumab) dosing, indications, interactions, adverse effects, and more. Medscape. 2021.
- Vu C, DeRonde K, Vega A, et al. Effects of Tocilizumab in COVID-19 patients: A cohort study. BMC Infect Dis 2020; 20.
- Somers EC, Eschenauer GA, Troost JP, et al. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clin Infect Dis 2021; 73:e445-e454.
- Nishimoto N, Terao K, Mima T, et al. Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, Tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood 2008; 112:3959-3964.
- Guaraldi G, Meschiari M, Cozzi-Lepri A, et al. Tocilizumab in patients with severe COVID.19: a retrospective cohort study. Lancet Rheumatol 2020; 2:e474-e484.
- Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with Tocilizumab. USA, Proc Natl Acad Sci 2020; 117:10970-10975.
- Rimland CA, Morgan CE, Bell GJ, et al. Clinical characteristics and early outcomes in patients with COVID-19 treated with Tocilizumab at a United States academic centre. MedRxiv 2020; 1-16.
- Campochiaro C, Della-Torre E, Cavalli G, et al. TOCI-RAF study group. Efficacy and safety of Tocilizumab in severe in COVID-19 patients: A single centre retrospective cohort study. Eur J Intern Med 2020; 76:43–49.
Author Info
Sonali Pathak* and Sarika Dakhode
Department of Community Medicine, Jawaharlal Nehru Medical College, Datta Meghe Institute of Medical Sciences, Sawangi (Meghe), Wardha, Maharashtra, IndiaCitation: Sonali Pathak, Sarika Dakhode, “Effect of Tocilizumab in Reducing Mortality and Need for Invasive Ventilation in a COVID-19 Infected Patients”, J Res Med Dent Sci, 2022, 10 (11): 025-029.
Received: 02-Sep-2022, Manuscript No. JRMDS-22-70676; , Pre QC No. JRMDS-22-70676(PQ); Editor assigned: 06-Sep-2022, Pre QC No. JRMDS-22-70676(PQ); Reviewed: 21-Sep-2022, QC No. JRMDS-22-70676; Revised: 03-Nov-2022, Manuscript No. JRMDS-22-70676(R); Published: 11-Nov-2022
