Research Article - (2025) Volume 13, Issue 1
Effect of Matricaria chamomilla Extract on Experimentally Induced Cutaneous Wound Healing in Rats
Nabaa Amer Nafea* and Ban A Ghani
*Correspondence: Nabaa Amer Nafea, Department of Oral Diagnosis, Oral Histology, College of Dentistry, University of Baghdad, Baghdad, Iraq, Email:
Abstract
Background: Wound healing is a complex and dynamic process of replacing devitalized and missing cellular structures and tissue layers. It is the interaction of a complex cascade of cellular events that generates resurfacing, reconstitution and restoration of the injured skin. There is increasing interest to use the medicinal plants in wound healing because of lower side effects and management of wounds over the years. Chamomile (Matricaria chamomilla L.) is a well-known medicinal plant species from the Asteraceae family and a commonly used natural remedy thought to be beneficial as a sedative, spasmolytic and anti-inflammatory agent.
Materials and methods: Thirty male albino rats (Rattus norvegicus albinus) of about 250-400 gm, were used in this study. Two circular full thickness wounds were made on the dorsum region of each animal with a sterile biopsy punch (5 mm diameter). Local Matricaria chamomilla essential oil application was done on wounds of the left side as experimental group, whereas the right side was left to heal spontaneously as control. The healing process was followed for the periods (3, 5 and 7 days) and specimens were prepared for histological analysis.
Results: Histological examination showed mean values of wound contraction decreased with time as lowest values were at day 7, highest mean values of inflammatory cell count was recorded in control groups at days 3 and 5, while epithelial thickness showed increased values with time in all groups.
Conclusion: The study revealed that local application of chamomile Matricaria chamomilla was effective in promoting wound healing process.
Keywords
Wound healing, Matricaria chamomilla, Rats, Complex and dynamic, Rattus norvegicus albinus
Introduction
Skin has the capacity to repair damaged by regeneration. This process is called “wound healing” and consists of coagulation, inflammation, proliferation and remodeling phases, in which a variety of cytokines and growth factors are, produced [1-4]. Both immune and non-immune cells, including macrophages, neutrophils, fibroblasts, vascular endothelial cells and keratinocytes are involved in this process.
Among the wide variety of medicinal herbs the Matricaria chamomilla, stands out and it has one of the most common uses of the herbal therapeutic forms. It has anti-inflammatory properties and antioxidant activity antispasmodic, sedative, anti-allergic, anti-hyperglycaemic and antimicrobial justifying the recognized use as medicinal herb [5,6].
Materials and Methods
Experimental design: The study was ethically approved by committee of college of dentistry/university of Baghdad. Thirty albino rats with a body weight of (250-400 gm) and 1-2 months of age were used in this study. All rats were maintained under controlled ventilation conditions, temperature, housing and feeding and were given a standard diet (pellet) with an easy access to the tap water. Animals were kept in standard separate cages and they were kept for 1 week in the same suitable environment and were fasted prior to operation for about 6-8 hours. The animals randomly divided into three main groups (10 rats in each) according to the healing intervals (3, 5 and 7 days). Two full thickness circular punch biopsy were made on the dorsum of each rat with sterile 5 mm punch biopsy instrument [7]. The wound at right side was left to heal spontaneously as control. Whereas the left side treated daily with Matricaria chamomilla essential oil.
Surgical procedures: Every animal was weighed in order to determine the dose of anesthesia required. The animal was placed on surgical table and general anesthesia was induced by intra muscular injection of xylazine 2% (0.4 mg/kg BW), plus ketamine HCL 50 mg (40 mg/kg BW). Removal of skin hair of the dorsal region was done. The skin was swept with 70% ethyl alcohol. Two full thickness skin wounds of circular area 2 mm depth were made on dorsal skin, distant 2.5 cm from each other, with a biopsy punch of 5 mm of diameter [8,9]. The two wounds were identified as right (control) was irrigated with distilled water and left 4 (experimental) locally treated with Matricaria chamomilla essential oil (10 μl) by micropipette. At the end of each healing period (3, 5 and 7 days) each rat was given anesthesia. The wound was lined including about 5 mm of the surrounding normal tissue and a full thickness skin was excised by surgical blade. The specimens were put in a plastic biopsy container filled with 10% formaldehyde for fixation.
Results
Histological findings of control group at wound site after three days duration shows remnant of necrotic tissue at surface, inflammatory cells infiltration in dermis (Figure 1). Microphotographs of wound site of experimental group shows complete epithelialization at surface, numerous new hair follicles, remodeling collagen fibers and fibroblasts (Figure 2). Microphotograph view of wound site of control group after 5 days shows wound surface sealed by new epithelium, developing hair follicles, remodeling collagen fibers (Figure 3). Other microphotograph of experimental group shows necrotic tissue at wound surface underlined by newly formed epithelium (Figure 4). After 7 days duration microphotograph view of wound site shows complete epithelialization, collagen fibers remodelling (Figure 5). Histological examination of wound of experimental group after 7 days shows complete epithelialization, wound site, hair follicles, remodeling collagen fibers (Figure 6).
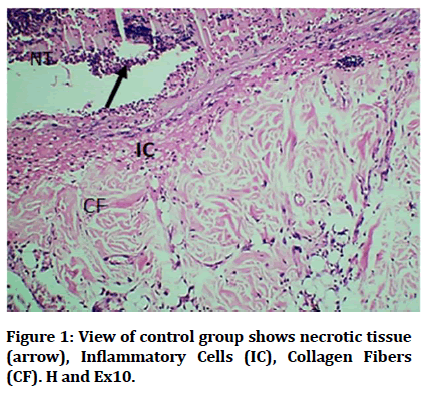
Figure 1: View of control group shows necrotic tissue (arrow), Inflammatory Cells (IC), Collagen Fibers (CF). H and Ex10.
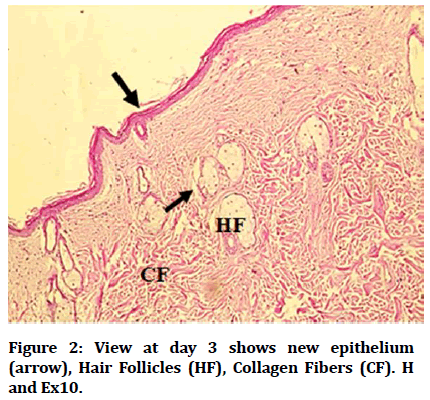
Figure 2: View at day 3 shows new epithelium (arrow), Hair Follicles (HF), Collagen Fibers (CF). H and Ex10.
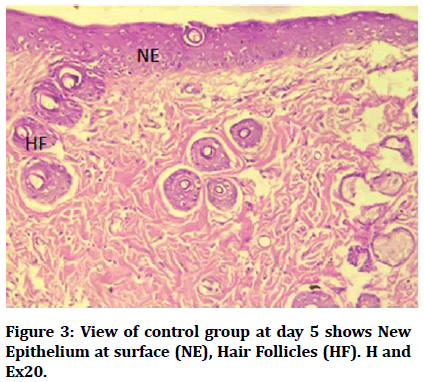
Figure 3: View of control group at day 5 shows New Epithelium at surface (NE), Hair Follicles (HF). H and Ex20.
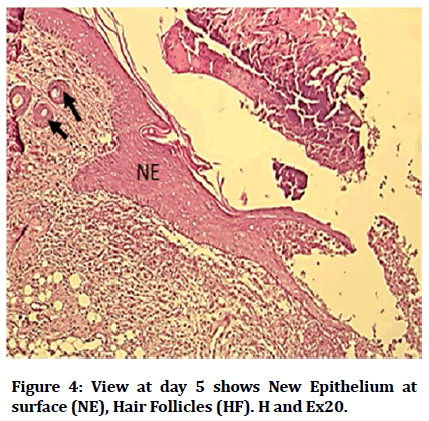
Figure 4: View at day 5 shows New Epithelium at surface (NE), Hair Follicles (HF). H and Ex20.
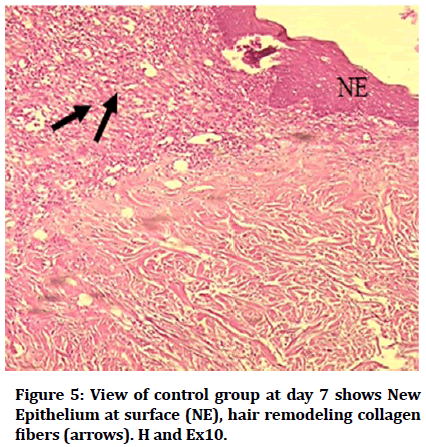
Figure 5: View of control group at day 7 shows New Epithelium at surface (NE), hair remodeling collagen fibers (arrows). H and Ex10.
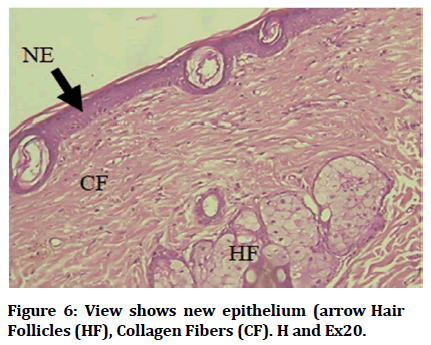
Figure 6: View shows new epithelium (arrow Hair Follicles (HF), Collagen Fibers (CF). H and Ex20.
Results revealed that the recorded mean values of wound contraction decreased with time in both control and experimental groups, high significant difference was recorded among different durations (Table 1 and Figure 7).
| Days | N | Wound size (mm) | Range | T-test | P-Value | |
|---|---|---|---|---|---|---|
| Experiment mean ± SD | Control mean ± SD | |||||
| Day 3 | 10 | 3.83 ± 0.37 | 4.27 ± 0.23 | 2.91-4.75 | 4.793 | 0.001 |
| Day 5 | 10 | 2.95 ± 0.35 | 3.84 ± 0.31 | 2.45-4.45 | 7.545 | 0.001 |
| Day 7 | 10 | 2.73 ± 0.24 | 3.69 ± 0.2 | 2.25-4.0 | 8.594 | 0.001 |
| *P>0.05 non-significant; **P ≤ 0.05 significant; ***p ≤ 0.01 highly significant | ||||||
Table 1: Descriptive statistics of wound contraction in (mm) for both groups in different durations.
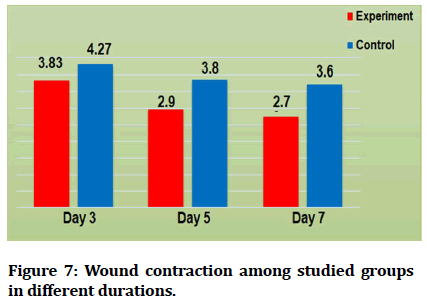
Figure 7: Wound contraction among studied groups in different durations.
Results revealed that mean values of inflammatory cell count decreased with time regarding experimental group. For control group the highest mean value recorded was at day 3 and the lowest value was at day 1 (Figure 8). Mean values of epithelial thickness parameter increased with time for both experimental and control groups in different durations. The highest mean values recorded at day 7 and the lowest mean values at day (Figure 9).
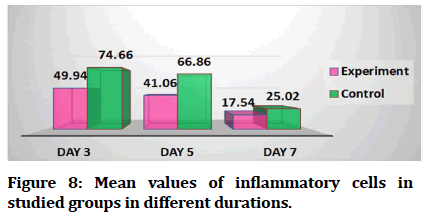
Figure 8: Mean values of inflammatory cells in studied groups in different durations.
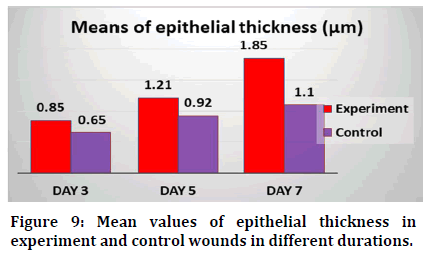
Figure 9: Mean values of epithelial thickness in experiment and control wounds in different durations.
Discussion
Skin wound healing is a dynamic and highly regulated process of cellular, humoral and molecular mechanisms which begins directly after wounding and might last for years. Every tissue disruption of normal anatomic structure with consecutive loss of function can be described as a wound [10]. Medicinal plants show wound healing effects by the different mechanisms, such as modulation in wound healing, decreasing bacterial count, improving collagen deposition, increasing fibroblasts and fibrocytes [11].
Rodents are often chosen for skin wound healing studies due to their readily accessibility, low price and small size, resulting in a more economical and effective use of restricted laboratory rooms and housing facilities [12].
Wound contraction is a healing response that functions to reduce the size of the tissue defect and subsequently decrease the amount of damaged tissue that needs repair. This response involves myofibroblasts, which are located in currently existing fibers and surrounding margins of the wound. These myofibroblasts function to pull newly formed collagen fibers in damaged tissues toward the center of the defect, thus reducing the size of the tissue defect wound contraction more rapid than epithelialization since new tissue is not created, causes a decrease in the healing time of rat wounds [13,14]. Healings begins with hemostasis at the site of injury, progresses to an inflammatory phase followed by proliferation of the epithelial and matrix components and ends with lying down of a highly organized collagn matrix [15]. During inflammation phase, the granulation tissue is composed predominantly of inflammatory cells, mainly neutrophils that are recruited to the wound site and removed during the progression of the repair process [16]. The proliferation phase starts about 3 days after wounding; it involves angiogenesis (by endothelial cells), granulation tissue formation by fibroblasts and re-epithelialization by keratinocytes. The fibroblasts produce a large amount of Extracellular Matrix (ECM), mainly collagen, to form the granulation tissue which replaces the damaged tissue. Meanwhile, the keratinocytes migrate, proliferate, differentiate and re-form a functional epidermis (re-epithelialization) [17]. The results of the present study, showed that percentage of wound contraction decreased in both groups with time, it was lowest at day 7 in experiment wound than that in controls in all days, in agreement with who found that, the area of wounds topically treated with lavender oil was significantly decreased as compared to that of wounds of control rats at 4, 6, 8 and 10 days after wounding [18].
In this study results showed that the mean values of inflammatory cells were lower in experimental groups than that in controls at days 3, 5 and 7 and the lowest mean values were at day 7 in experiment group. In agreement with findings of who study effect of essential oil extracted from Eugenia dysenterica DC (Myrtaceae) leaves on wound healing [19].
Also agrees with results concerned with inflammatory cells scoring which were obtained by who studied the effect of Brazilian green propolis in tissue repair of cutaneous wounds in wistar rats and they found that at days 1 and 3 the treated wounds demonstrated significant bigger means for inflammatory cells. The decrease in inflammatory cell mean values with time could be explained according to studies by who reported that fenugreek have antioxidant properties which can accelerate process of wound healing. Epithelial thickness between experimental and control wounds at days 3, 5 and 7 [20,21].
In this study, mean of epithelial thickness was significantly increased in experimental and control groups with time and highest values detected at day 7 in experimental group. In line with it was observed at day 3 post-wounding the treatment with 20% Essential Oil of Croton zehntneri and trans-anethole (EOCz) reduced the swelling and exudates, accelerated the wound closure, with an enhanced number of fibroblasts and collagen fibres in treated mice demonstrating its wound healing activity [22].
Conclusion
Histological examination showed mean values of wound contraction decreased with time as lowest values were at day 7, highest mean values of inflammatory cell count was recorded in control groups at days 3 and 5, while epithelial thickness showed increased values with time in all groups. The obtained results indicated that using Matricaria chamomilla extract have potential activity in enhancing cutaneous wound healing in rats.
References
- Robson MC. Wound healing: Biologic features and approaches to maximize healing trajectories. Curr Problems Surg 2001; 38:61-140.
[Crossref] [Google Scholar] [PubMed]
- Mast BA, Schultz GS. Interactions of cytokines, growth factors and proteases in acute and chronic wounds. Wound Repair Regen 1996; 4:411-420.
[Crossref] [Google Scholar] [PubMed]
- Rumalla VK, Borah GL. Cytokines, growth factors and plastic surgery. Plast Reconstr Surg 2001; 108:719-733.
[Crossref] [Google Scholar] [PubMed]
- Barrientos S, Stojadinovic O, Golinko MS, et al. Growth factors and cytokines in wound healing. Wound Repair Regen 2008; 16:585-601.
[Crossref] [Google Scholar] [PubMed]
- Srivastava JK, Shankar E, Gupta S. Chamomile: A herbal medicine of the past with a bright future. Mol Med Rep 2010; 3:895-901.
[Crossref] [Google Scholar] [PubMed]
- Haghi G, Hatami A, Safaei A, et al. Analysis of phenolic compounds in Matricaria chamomilla and its extracts by UPLC-UV. Res Pharm Sci 2014; 9:31-37.
[Google Scholar] [PubMed]
- Farahpour MR, Habibi M. Evaluation of the wound healing activity of an ethanolic extract of ceylon cinnamon in mice. Veterinarni Med 2012; 57:53–57.
- Moreira CF, Vieira PC, Silva MF. Skin wound healing model excisional wounding and assessment of lesion area. Bio-protocol 2015; 22:e1661.
- Liu H, Lin S, Xiao D, et al. Evaluation of the wound healing potential of Resina Draconis (Dracaena cochinchinensis) in animal models. Evid Based Complement Alternat Med 2013; 2013.
[Crossref] [Google Scholar] [PubMed]
- Lazarus GS, Cooper DM, Knighton DR, et al. Definitions and guidelines for assessment of wounds and evaluation of healing. Wound Repair Regen 1994; 2:165-170.
[Crossref] [Google Scholar] [PubMed]
- Raina R, Prawez S, Verma PK, et al. Medicinal plants and their role in wound healing. Vet Scan 2008; 3:1-24.
- Dorsett-Martin WA, Wysocki AB. Humana Press Inc. Models of skin wound healing. Rat models of skin wound healing. Sourcebook Models Biomed Res 2008; 65:633-638.
- Michelle M, Lesperance MS, Travis L, et al. Postsurgical orthopedic sports rehabilitation knee and shoulder. 2006; 3-18.
- Mogford JE, Mustoe TA. Experimental models of wound healing. In: Falanga V, Ed. Cutaneous wound healing. London: Martin Dunitz Ltd., 2001; 109-122.
- Velnar T, Bailey T, Smrkolj V. The wound healing process: An overview of the cellular and molecular mechanisms. J Int Med Res 2009; 37:1528-1542.
[Crossref] [Google Scholar] [PubMed]
- Gurtner GC, Werner S, Barrandon Y, et al. Wound repair and regeneration. Nature 2008; 453:314-321.
[Crossref] [Google Scholar] [PubMed]
- Shedoeva A, Leavesley D, Upton Z, et al. Wound healing and the use of medicinal plants. Evid Based Complement Alternat Med 2019.
[Crossref] [Google Scholar] [PubMed]
- Mori HM, Kawanami H, Kawahata H, et al. Wound healing potential of lavender oil by acceleration of granulation and wound contraction through induction of TGF-β in a rat model. BMC Complement Altern Med 2016; 16:1-1.
[Crossref] [Google Scholar] [PubMed]
- Mazutti da Silva SM, Rezende Costa CR, Martins Gelfuso G, et al. Wound healing effect of essential oil extracted from Eugenia dysenterica DC (Myrtaceae) leaves. Molecules 2018; 24:2.
[Crossref] [Google Scholar] [PubMed]
- Marcos CS, Paulo HC, Alice GP, et al. Histological evaluation on Brazilian green propolis effect in tissue repair of wistar rats cutaneous wounds. Latin American J Pharm 2011; 30:383-387.
- Kaur C, Kapoor HC. Anti-oxidant activity and total phenolic content of some Asian vegetables. Int J Food Sci Technol 2002; 37:153-161.
- Cavalcanti JM, Leal-Cardoso JH, Diniz LR, et al. The essential oil of croton zehntneri and trans-anethole improves cutaneous wound healing. J Ethnopharmacol 2012; 144:240-247.
[Crossref] [Google Scholar] [PubMed]
Author Info
Nabaa Amer Nafea* and Ban A Ghani
Department of Oral Diagnosis, Oral Histology, College of Dentistry, University of Baghdad, Baghdad, IraqCitation: Nabaa Amer Nafea, Ban A Ghani, Effect of Matricaria chamomilla Extract on Experimentally Induced Cutaneous Wound Healing in Rats, J Res Med Dent Sci, 2023, 11 (06): 001-005.
Received: 30-Apr-2022, Manuscript No. JRMDS-22-62362; , Pre QC No. JRMDS-22-62362; Editor assigned: 03-Apr-2022, Pre QC No. JRMDS-22-62362; Reviewed: 18-May-2022, QC No. JRMDS-22-62362; Revised: 25-May-2023, Manuscript No. JRMDS-22-62362; Published: 05-Jun-2023
