Research - (2020) Advances in Dental Surgery
Different Orthodontic Adhesive Systems and Enamel Demineralization Around Metal Brackets Assessed by a Laser Fluorescence Device (A Comparative an in-vitro study)
Ahmed Dhiaa Hatf1* and Mustafa M AL-Khatieeb2
*Correspondence: Ahmed Dhiaa Hatf, Ministry of Iraqi Health, Iraq, Email:
Abstract
regarding the enamel demineralization using a laser fluorescence device (DIAGNOdent™ pen).
Materials and methods: Eighty human upper premolar teeth were randomly divided into two groups (40 teeth each); the first group in which the bonded teeth were stored in distilled water for 30 days at 37°C, and the second group in which the bonded teeth were subjected to acid challenge. Each group was subdivided in four subgroups (10 teeth each) according to the type of adhesive system that would be bonded to metal brackets either non-fluoride releasing adhesive (NFRA), fluoride releasing adhesive (FRA), Fluoride releasing bond with self-etching primer (FRBSP), or powder and liquid orthodontic fluoride releasing adhesive (PLFRA). After 30 days, the evaluation of enamel demineralization was performed by laser fluorescence device (DIAGNOdent™ Pen).
Result: There were highly significant differences in the means of fluorescence variation values (ΔFV) among all the four tested adhesive systems in water storage and acid challenge groups using ANOVA F-test. In both groups, the NFRA subgroup exhibited a highest fluorescence variation value, followed by FRASP subgroup, then FRA subgroup, while the PLFRA subgroup had a lowest value of fluorescence variation, indicating less enamel demineralization around the bracket. The independent t-test showed highly significant differences in enamel demineralization between water storage and acid challenge groups.
Conclusion: The PLFRA showed the least enamel demineralization around the bracket compared with other adhesive systems. So, it should be advocated for any orthodontic patient with high caries risk or diminished salivary pH in order to reduce or remineralize the enamel demineralization around the bracket.
Keywords
Enamel demineralization, Fluoride releasing adhesive, Acid challenge, Laser fluorescence
Introduction
The fixed orthodontic appliances are still associated with a high risk of forming the white spot lesions (WSLs) even with the improvements in materials and treatment mechanics [1]. The prevalence of the WSLs during the orthodontic therapy expressed to a range from 13% to 75%. The maintenance of oral hygiene is impeded by components of fixed orthodontic appliances, which encourage the plaque accumulation around the bracket base. These lesions can occur through a short duration about 4 weeks, which is usually within the intervals of orthodontic treatment appointments [2].The Prevention of the WSLs must be the first goal of an orthodontist. Accordingly, the most essential way for averting WSL development is the patient education and motivation. The other means have been utilized for reducing the extent of WSLs are dentifrice, mouthwash, gels, and varnishes, all are formulated with fluoride [3].In restorative dentistry, the fluoride-releasing bonding system, Clearfil Liner Bond F (Kuraray Medical Inc, Okayama, Japan) had been advanced. It contained a specially treated sodium fluoride (NaF), which was effective in reducing the demineralization while maintaining the bonding strength [4].
The Light Bond paste and sealant had been developed as polyacid modified composite resins, with a patented monomer of fluoridereleasing property [5]. The Resin modified glass ionomer cements (RMGIC) had been combined with the preferable properties of composite resin including the shear bond strength and fluoridereleasing feature of glass ionomer cement. Several RMGICs had been proved for reducing enamel demineralization, one of them, Fuji Ortho LC (GC Company, Tokyo, Japan) [6]. DIAGNOdent pen 2190 (KAVO, Biberach, Germany) was used for the detection of enamel demineralization around the bracket base by applying the red fluorescence in caries lesions. The red fluorescence was excited by a 655-nm laser. The intensity of detected red fluorescence was about 700 nm and converted into a value between 0 and 99. Van der Veen [7] reported that the laser fluorescence device can be utilized easily for the occlusal and interproximal surfaces of the teeth and equally applied to evaluate the carious lesions on the smooth surfaces. The objective of present study was to evaluate the effectiveness of the different orthodontic adhesive systems regarding the enamel demineralization using a laser fluorescence device (DIAGNOdent™ pen).
Materials and Methods
After inspection of 138 human upper first premolars extracted for orthodontic purposes, only 80 teeth were involved, that had an intact buccal surface and free from caries, restorations, cracks, and fluorosis and not subjected to any chemical treatment. They stored in 1% chloramine-T solution for a week and subsequently, they saved in distilled water until conducting the bonding procedures [8]. The teeth were divided in two groups (40 teeth each); the first group in which the bonded teeth were saved in distilled water for 30 days at 37°C and the second group in which the bonded teeth were subjected to acid challenge. Each group was subdivided in four subgroups (10 teeth each) and mounted in auto-polymerized acrylic blocks before bonding. The acrylic blocks were coded to facilitate the randomization procedure.
Brackets
Eighty upper first premolar stainless-steel brackets of Discovery® Smart type (Dentaurum company, Ispringen, Germany) were used in this study. The prescription of upper premolar bracket was MBT system with slot size 0.022×0.030 of an inch and the bracket’s bonding surface area is 10.56 mm2.
Sample preparation
The polishing of buccal tooth surfaces was done with brushes and non-fluoride containing pumice for 10 seconds and then, washed and dried for other 10 seconds [9]. A portion of adhesive tape (7x7 mm) was positioned on the buccal tooth surfaces. A nail varnish (Flormar, Turkey) was used to cover the remaining tooth surfaces two times with a 3-hours period. The adhesive tape was removed after 24 hours, and a cotton pellet dipped in alcohol was used to eliminate any residual adhesive. The tooth surface was examined under the stereomicroscope (10 X) to ensure that there is no residual adhesive [10].
Bonding procedures
At room temperature, the bonding procedure was performed by one of the four bonding procedures according to the manufacture instructions as followed:
1.Non fluoride releasing adhesive: The enamel surfaces were etched with 37% phosphoric acid etching gel (Perfect Etch-E, Perfection Plus, UK) for 30 seconds, then washed for 10 seconds and air-dried gently. A thin film of Transbond XT primer was applied to the etched enamel surfaces, then polymerized by a LED light curing unit (O-light, Woodpecker, China) for 10 se¬conds.
2.Fluoride releasing adhesive: The teeth were bonded with Light Bond paste and sealant (Reliance Orthodontic Products, Itasca, Illinois, USA). The liquid etchant (37% phosphoric acid) was applied to the buccal tooth surfaces for 30 seconds, then washed for 30 seconds and airdried gently. The fluoride releasing sealant resin was painted with a disposable brush in a thin uniform coating, followed by mild air-drying, and then cured for 30 seconds [5].
3. Fluoride releasing bond with self-etching primer: The teeth were bonded with Clearfil Liner Bond F (Kuraray Noritake Dental Inc., Okayama, Japan) and Transbond XT. The selfetching primer was applied for 20 seconds, then dried with a mild air flow. The Clearfil Liner Bond F which had fluoride releasing property, was applied, gently air flowed to create a uniform bond film, and light cured for 10 seconds [11]
4. Powder and liquid orthodontic fluoride releasing adhesive: the teeth were bonded with Fuji Ortho LC (GC Company, Tokyo, Japan). The etching gel (37% phosphoric acid) was applied for 30 seconds, then washed for 10 seconds. The bonding area was not completely desiccated through the bonding procedure. The cement was prepared by one scoop of powder and two drops of liquid on a mixing pad using a plastic spatula to achieve a glossy consistency [10].
In the four bonding procedures, the bracket base was coated with an adhesive paste or cement, and placed at the center of the buccal tooth surface. A load (200 gm) was placed on each bracket using a surveyor for 10 seconds to achieve uniform adhesive thickness [9]. Any excess of adhesive was removed by dental explorer before the curing. The LED light curing unit with curing intensity 1200 mw/cm² was applied for 40 seconds (10 seconds from each side of bracket) [12].
Once the bonding procedures were completed, the bonded teeth of first group were stored in the incubator in deionized water inside sealed containers at 37°C for 30 days with daily refreshment, in order to avoid the cumulative effects [13,14]. While the bonded teeth in the second group stored in deionized water for 24 hours at 37°C prior to the acidic challenge experiment. The acidic solution (pH=2.5) of 500 ml was prepared by gradual addition of 1.5 ml of HCl [1M] in distilled water. The acidic challenge was performed by immersing the samples in the acidic solution through a protocol of three session per day, 5 min each, with equal intervening periods (2 hour) for 30 days. The samples were stored in distilled water (pH=6) at 37°C for the rest of day in order to mimic the wet oral environment. After each session, each storage medium was periodically renewed, and before and after each session, the samples were washed and air dried [14].
Laser fluorescence measurements
The DIAGNOdent™ Pen 2190 was used with a sapphire fissure probe in this study to detect the enamel demineralization around the bracket bases in both groups (water storage and acid challenge groups) (Figure 1). According to the manufacture instructions, the calibration of DIAGNOdent™ pen was performed against its own ceramic standard disc and a zero-baseline fluorescence was achieved using the sound smooth surface of the tooth. The calibration was rechecked before examining each ten bonded teeth [15]. The initial measurements were recorded at the time of the bracket bonding to determine a baseline fluorescence value of each bonded tooth, which was referred as FV1. The Laser fluorescence measurements were obtained on the buccal surfaces at 1 mm away from the middle of the mesial, distal, gingival, and occlusal margins of the brackets for three times at each aspect and the mean value was taken [16]. The mean value of four readings (occlusal, gingival, mesial and distal) was obtained for each bonded tooth [17]. Each site was air-dried for 5 sec and the tip of DIAGNOdent™ Pen was held perpendicular against the tooth surface with circular tilted movements to collect the fluorescence from all directions [15] (Figure 2). After 30 days of samples storage either in distilled water or in the acidic environment, the final DIAGNOdent™ Pen measurements were also recorded for each bonded tooth which was referred as FV2 (in the same maneuver of FV1). The fluorescence variation (ΔFV) was determined as the change from initial fluorescence value (FV1) to the final fluorescence value (FV2) and recorded after demineralization process in both groups.
Figure 1. A set of laser fluorescence device (KAVO, Biberach, Germany).
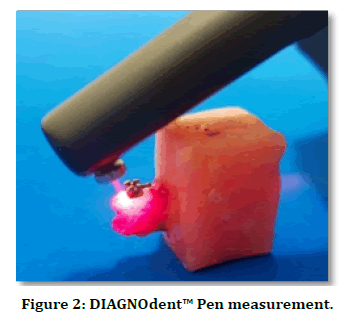
Figure 2. DIAGNOdent™ Pen measurement.
Statistical analysis
The collected data was analyzed using SPSS (version 25.0, SPSS Inc. Illinois, USA) by Oneway analysis of variance (ANOVA), Post-hoc Tukey’s HSD test, and Independent t-test. For statistical evaluation, the levels of significance were used; a non-significant difference (NS) where the P > 0.05, a significant difference (S) where 0.05 ≥ P>0.01, and a highly significant difference (HS) where P ≤ 0.01. With respect to the intra- and inter-examiner reliability, the DIAGNOdent™ pen readings were done at ten bonded teeth by one examiner with 10 days interval and by two examiners at same time. The intraclass correlation coefficient (ICC) showed excellent reliability for intra- and inter-examiner calibration, as shown in table 1.
| Calibration | No. of samples | ICC | 95% CI |
|---|---|---|---|
| Intra-examiner | 10 | 0.934 | 0.730- 0.984 |
| Inter-examiner | 10 | 0.973 | 0.897- 0.993 |
Table 1: Shows Intraclass Correlation Coefficient (ICC) to assess the intra-examiner and inter-examiner reliability of DIAGNOdent™ pen readings.
Results
Table 2 showed the mean, standard deviation (S.D.), standard error (S.E.) minimum (Min.), and maximum (Max.) values of fluorescence variation (ΔFV) in both groups (water storage and acid challenge). In water storage, the highest mean value of ΔFV was in NFRA group (11.075 ± 1.363), followed by that of FRBSP group (9.775 ± 0.606), then FRA group (9.641 ± 1.191), and lastly the PLFRA group, which had the lowest mean of ΔFV (8.317 ± 0.787). In acid challenge group, the highest mean value of ΔFV was in NFRA group (13.924 ± 0.885), followed by that of FRBSP group (13.158 ± 0.638), then FRA group (13.041 ± 1.154), and lastly the PLFRA group, which had the lowest mean of ΔFV (11.908 ± 0.894). Table 3 showed the comparison of mean differences of fluorescence variation (ΔFV) within both ageing groups between all adhesive systems. The Oneway analysis of variance (ANOVA) revealed that there were highly significant differences between all adhesive systems in both groups of ageing media.
| Group | Adhesive system | N | Mean (ΔFV) | S.D. | S. E. | Min. | Max. |
|---|---|---|---|---|---|---|---|
| Water storage | NFRA | 10 | 11.075 | 1.363 | 0.43 | 9.75 | 14.25 |
| FRA | 10 | 9.641 | 1.191 | 0.377 | 8.25 | 12.418 | |
| FRBSP | 10 | 9.775 | 0.607 | 0.192 | 8.835 | 10.583 | |
| PLFRA | 10 | 8.317 | 0.787 | 0.249 | 7.165 | 9.25 | |
| Acid challenge | NFRA | 10 | 13.924 | 0.885 | 0.28 | 12.5 | 15.168 |
| FRA | 10 | 13.041 | 1.154 | 0.365 | 11.33 | 15.157 | |
| FRBSP | 10 | 13.158 | 0.638 | 0.202 | 11.668 | 14.085 | |
| PLFRA | 10 | 11.908 | 0.0894 | 0.283 | 10.748 | 13.583 |
Table 2: Descriptive statistics of the laser fluorescence test of different adhesive groups.
| Group | Adhesive system | Comparison | |||
|---|---|---|---|---|---|
| ANOVA test | Tukey’s HSD test | ||||
| F-test | p-value | Between Subgroups |
p-value | ||
| Water storage | NFRA | 11.93 | 0 | NFRA-FRA | 0.018 (S) |
| FRA | NFRA-FRBSP | 0.038 (S) | |||
| FRSBP | NFRA-PLFRA | 0.000 (HS) | |||
| PLFRA | FRA-FRBSP | 0.991 (NS) | |||
| FRA-PLFRA | 0.033 (S) | ||||
| FRBSP-PLFRA | 0.016 (S) | ||||
| Acid challenge | NFRA | 8.319 | 0 | NFRA-FRA | 0.153 (NS) |
| FRA | NFRA-FRBSP | 0.255 (NS) | |||
| FRSBP | NFRA-PLFRA | 0.000 (HS) | |||
| PLFRA | FRA-FRBSP | 0.992 (NS) | |||
| FRA-PLFRA | 0.041 (S) | ||||
| FRBSP-PLFRA | 0.020 (S) | ||||
Table 3: Comparison of the laser fluorescence test in different groups by ANOVA and Post-hoc Tukey’s HSD test.
In water storage group, the Post-hoc Tukey’s HSD test revealed that there was highly significant difference between NFRA and PLFRA groups, while there were significant differences between NFRA and FRA, NFRA and FRBSP, FRA and PLFRA, and FRBSP and PLFRA groups, and there was non-significant difference between FRA and FRBSP groups. In acid challenge group, the Posthoc Tukey’s HSD test revealed that there was a highly significant difference between NFRA and PLFRA groups, while there were significant differences between FRA and PLFRA, FRBSP and PLFRA and, there were no significant differences between NFRA and FRA, NFRA and FRBSP, and FRA and FRBSP groups.The effect the ageing media (water storage and acid challenge) on the enamel demineralization associated with the four test adhesive systems was determined by the independent t-test. The results observed that there were highly significant differences regarding the enamel demineralization between the water storage and acid challenge groups, as shown in Table 4.
| Adhesive system | Group | Comparison | ||
|---|---|---|---|---|
| Mean Differences | t-value | P-value | ||
| NFRA | Water storage | 2.848 | 5.544 | 0.000 (HS) |
| Acid challenge | ||||
| FRA | Water storage | 3.4 | 6.484 | 0.000 (HS) |
| Acid challenge | ||||
| FRBSP | Water storage | 3.383 | 12.15 | 0.000 (HS) |
| Acid challenge | ||||
| PLFRA | Water storage | 3.591 | 9.538 | 0.000 (HS) |
| Acid challenge | ||||
Table 4: Comparison of ageing media on the enamel demineralization associated with the four test adhesive systems using independent t-test.
Discussion
The enamel demineralization during the orthodontic therapy occurred as the components of fixed orthodontic appliances provide harboring areas for plaque accumulation [18]. Also, the frequent carbohydrate consumption and increased consumption of acidic soft drinks had a critical impact on enamel demineralization during the orthodontic treatment [19].
The early detection of the WSLs during orthodontic therapy is beneficial in lessening its progression, which can be achieved easily and efficiently by using a laser fluorescence device, DIAGNOdent™ Pen 2190 [16,20].
In order to imitate a clinical situation, the acid challenge protocol was followed in present study assuming a patient with fixed orthodontic therapy taking a highly acidic drink (pH 2.5) 3-times per day with a 5 minutes drinking period for a duration of 30 days, this period is enough to inspect the enamel demineralization/ remineralization potential in vitro condition. The water storage of bonded teeth was followed for 30 days at 37°C to exclude any possible effect of prolonged water storage on the enamel demineralization and bonding strength, accompanying the acidic attack [14].
In the current study, the DIAGNOdent™ Pen 2190 was used to detect the demineralization around the metal bracket. The device was worked in easy and reproducible manner. Shi et al. [21] reported in his in-vitro study that there was positive correlation (0.8) between the lesion depth assessment (the gold standard) and the DIAGNOdent™ pen readings. They concluded that the DIAGNOdent™ pen could be a valuable clinical diagnostic tool in assessing the smoothsurface carious lesions.
According to the results of the current study, there were statistically significant differences among the tested adhesive systems in both groups (water storage and acid challenge).
Regarding the water storage group, no previous studies evaluated the influence of prolonged water storage on the effectiveness of fluoride-releasing adhesive systems in reducing the enamel demineralization. The laser fluorescence test showed that the highest mean of fluorescence variation (ΔFV) was in NFRA group (11.075 ± 1.363), indicating more enamel demineralization around the bracket, while the least mean of ΔFV was in PLFRA group (8.317 ± 0.787), the present study observed that there was highly significant difference between these two groups, this can be attributed to fact that the Fuji Ortho LC releases greater amount of fluoride over the long period [13]. The FRBSP group had less ΔFV mean (9.775 ± 0.606) than NFRA group, the difference between these two groups was significant, this can be attributed to the presence of the specially treated sodium fluoride (NaF) within the composition of Clearfil Liner Bond F of the FRBSP system, which is effective in reducing the demineralization and also, it has been shown that self-etching primers in the produced less aggressive enamel demineralization in comparing with the conventual acid etching under the regular conditions [22,23]. The FRA group has less ΔFV mean (9.641 ± 1.191) than the NFRA group, and the difference between them was significant, this can be attributed to the presence of a patented fluoride-releasing monomer in the Light Bond paste and sealant [4]. Finally, the current observation found that there were significant differences between FRA and PLFRA, and FRBSP and PLFRA groups, on other hand a non-significant difference was found between FRA and FRBSP groups because both FRA and FRBSP had the ability of fluoride releasing property.
Regarding acid challenge group, the laser fluorescence test showed that the highest mean of ΔFV was in NFRA group (13.924 ± 0.885) and the least mean value of ΔFV in the PLFRA group (11.908 ± 0.894), indicating less amount of enamel demineralization around the bracket among other tested adhesive systems. The current finding observed that there was highly significant difference between NFRA and PLFRA groups, this related to fact that the fluoride releasing ability of RMGICs (in PLFRA group) increases as the pH decreases by the effect of the chemical erosion and solubility of cement surface as reported by Gandolfi et al. [24], these outcomes supported by Uysal et al. [20], who used the DIAGNOdent™ pen for assessment the enamel demineralization around metal brackets, which bonded with Aegis Ortho, Fuji Ortho LC and Transbond XT, undergoing pH-cycling for 21 days and found that the Fuji Ortho LC significantly reduced the enamel demineralization. These findings supported by most studies in the literatures [6,25,26].
The mean of ΔFV was less in FRBSP group (13.158 ± 0.638) than NFRA (13.924 ± 0.885), and the difference between these two groups was non-significant, this was related to the acid challenge protocol that followed in current study and also, the amount of daily fluoride released by FRBSP had been decreased over time [23].
Many previous studies evaluated the Clearfil Protect Bond as fluoride releasing bond with self-etching primer, which have nearly the same components of Clearfil Liner Bond F that was used in the current study, both are manufactured by kuraray medical company and have fluoridereleasing property. Our study results were agreed with Paschos et al. [27], who assessed in his randomized clinical trials, the effect of different fluoride releasing self-etching primers in prevention of enamel demineralization using the laser fluorescence device (DIAGNOdent™) and concluded that there was no benefit for Clearfil protect bond in prevention of enamel demineralization.
The mean value of ΔFV was less in FRA group (13.041 ± 1.154) than NFRA group, but the difference between them was a non-significant. These results were congruent with Chin et al. [28], who evaluated the effect of daily acidic attack on the fluoride release profile and lesion depth of different orthodontic adhesives and found that extensive erosion occurred in peri-bracket area in samples bonded with the Transbond XT and Light Bond.
Finally, the current study showed that there were significant differences between the PLFRA and FRA, and PLFRA and FRBSP groups, these results agreed with Paschos et al. [11], who found that the lesion depth was more for fluoride releasing composite resins than for RMGIC (Fuji Ortho LC).
In regard to the effect of ageing media on enamel demineralization in vicinity of the bracket, our study findings observed that there were highly significant differences between the water storage and acid challenge groups with high fluorescence variation values in latter group. These results can match with Oncag et al. [29], who evaluated the effect of acidic drinks on enamel demineralization around orthodontic bracket using the scanning electronic microscope and observed that the control group (artificial saliva) exhibited healthier enamel surface in comparison to the experimental groups that showed more erosion on the enamel surface.
Clinical Considerations and Conclusion
According to the results of current study:
All fluoride releasing adhesive systems worked well in water storage condition compared to non-fluoride release adhesive system. The type of ageing media significantly affected the efficiency of the fluoride releasing adhesive system in reducing the enamel demineralization.
In acidic challenge experiment, only the PLFRA exhibited significant reduction of enamel demineralization adjacent to the bracket in comparison to other tested adhesive systems. So, it can be concluded that the PLFRA system (Fuji Ortho LC adhesive) was the best among other adhesive systems in reduction of demineralization process.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Wenderoth CJ, Weinstein M, Borislow AI. Effectiveness of a fluoride-releasing sealant in reducing decalcification during orthodontic treatment. Am J Orthod Dentofacial Orthop 1999; 116:629-634.
- Maxfield BJ, Hamdan AM, Tüfekçi E, et al. Development of white spot lesions during orthodontic treatment: perceptions of patients, parents, orthodontists, and general dentists. Am J Orthod Dentofacial Orthop 2012; 141:337-344.
- Kucuk EB, Malkoc S, Demir A. Microcomputed tomography evaluation of white spot lesion remineralization with various procedures. Am J Orthod Dentofac Orthop 2016; 150:483–490.
- Alkis H, Turkkahraman H, Adanir N. Microleakage under orthodontic brackets bonded with different adhesive systems. European J Dent 2015; 9:117-21.
- Pseiner BC, Freudenthaler J, Jonke E, et al. Shear bond strength of fluoride-releasing orthodontic bonding and composite materials. Eur J Orthodont 2010; 32:268-273.
- Sudjalim TR, Woods MG, Manton DJ, et al. Prevention of demineralization around orthodontic brackets in vitro. Am J Orthodont Dentofacial Orthop 2007; 131:705-e1.
- Van der Veen MH. Detecting short-term changes in the activity of caries lesions with the aid of new technologies. Current Oral Health Reports 2015; 2:102-109.
- Korbmacher HM, Huck L, Kahl-Nieke B. Fluoride-releasing adhesive and antimicrobial self-etching primer effects on shear bond strength of orthodontic brackets. Angle Orthodont 2006; 76:845-850.
- Bishara SE, Ostby AW, Ajlouni R, et al. A new premixed self-etch adhesive for bonding orthodontic brackets. Angle Orthod 2008; 78:1101-1104.
- Shirazi M, Tamadon M, Izadi M. Effect of addition of bioactive glass to resin modified glass ionomer cement on enamel demineralization under orthodontic brackets. J Clin Exp Dent 2019; 11:e521.
- Paschos E, Kleinschrodt T, Clementino-Luedemann T, et al. Effect of different bonding agents on prevention of enamel demineralization around orthodontic brackets. Am J Orthodont Dent Orthop 2009; 135:603-612.
- Al-Khatieeb MM, Mohammed SA, Attar AMA. Evaluation of a new orthodontic bonding system (Beauty Ortho Bond). J Baghdad College Dent 2015; 27:175-181.
- McNeill CJ, Wiltshire WA, Dawes C, et al. Fluoride release from new light-cured orthodontic bonding agents. Am J Orthodont Dentofac Orthop 2001; 120:392-397.
- Ibrahim AI, Al-Hasani NR, Thompson VP, et al. In vitro bond strengths post thermal and fatigue load cycling of sapphire brackets bonded with self-etch primer and evaluation of enamel damage. J Clin Exp Dent 2020; 12:e22.
- Bahrololoomi Z, Musavi SA, Kabudan M. In vitro evaluation of the efficacy of laser fluorescence (DIAGNOdent) to detect demineralization and remineralization of smooth enamel lesions. J Conserv Dent 2013; 16:362–366.
- Comert S, Oz AA. Clinical effect of a fluoride-releasing and rechargeable primer in reducing white spot lesions during orthodontic treatment. Am J Orthodont Dentofacial Orthop 2020; 157:67-72.
- Tawfik MA, Mehesen R, Maaly T. Evaluation of modified glass ionomer cements with protein repellent and nanostructured antibacterial properties in prevention of enamel demineralization. An in vivo study. Egyptian Dent J 2019; 65:959-964.
- Mount GJ, Hume WR. Preservation and restoration of tooth structure. 2nd Edn Queensland, Australia: Knowledge Books and Software 2005; 61-82.
- Chang HS, Walsh L, Freer TJ. Enamel demineralization during orthodontic treatment. Aetiology and prevention. Australian Dent J 1997; 42:322-327.
- Uysal T, Amasyali M, Koyuturk AE, et al. Efficiency of amorphous calcium phosphate–containing orthodontic composite and resin modified glass ionomer on demineralization evaluated by a new laser fluorescence device. Europ J Dent 2009; 3:127-134.
- Shi XQ, Tranæus S, Angmar-Månsson B. Validation of DIAGNO dent for quantification of smooth-surface caries: An in vitro study. Acta Odontologica Scandinavica 2001; 59:74-78.
- Pirmoradian M, Esmailzadeh S, Davaie S, et al. Resistance to demineralization of adjacent enamel and dentine, fluoride release and dentine bond strength of fluoride-containing self-etch adhesive systems. J Clin Exp Dent 2020; 12:e381.
- Cal-Neto JP, Miguel JAM. Scanning electron microscopy evaluation of the bonding mechanism of a self-etching primer on enamel. Angle Orthodont 2006; 76:132-136.
- Gandolfi MG, Chersoni S, Acquaviva GL, et al. Fluoride release and absorption at different pH from glass-ionomer cements. Dent Materials 2006; 22:441-449.
- Ashith MV. Comparitive evaluation of enamel demineralization using conventional light cure composite resin and RMGIC-An In vivo Study. Int J Preventive Clin Dent Res 2015; 2:15-22.
- Mohamed MH, Refaat WE, Morcos SS. Effect of different fluoride releasing bonding agents in preventing of enamel demineralization around orthodontic brackets. Egyptian Orthodont J 2020; 50:13-34.
- Paschos E, Kurochkina N, Huth KC, et al. Failure rate of brackets bonded with antimicrobial and fluoride-releasing, self-etching primer and the effect on prevention of enamel demineralization. Am J Orthodont Dent Orthop 2009; 135:613–620.
- Chin MY, Sandham A, Rumachik EN, et al. Fluoride release and cariostatic potential of orthodontic adhesives with and without daily fluoride rinsing. Am J Orthodont Dent Orthop 2009; 136:547-553.
- Oncag G, Tuncer AV, Tosun, YS. Acidic soft drinks effects on the shear bond strength of orthodontic brackets and a scanning electron microscopy evaluation of the enamel. Angle Orthodont 2005; 75:247-253.
Author Info
Ahmed Dhiaa Hatf1* and Mustafa M AL-Khatieeb2
1Ministry of Iraqi Health, Baghdad, Iraq2Department of Orthodontics, College of Dentistry, University of Baghdad, Iraq
Citation: Ahmed DhiaaHatf, Mustafa M AL-Khatieeb, Different Orthodontic Adhesive Systems and Enamel Demineralization Around Metal Brackets Assessed by a Laser Fluorescence Device (A Comparative an in-vitro study), J Res Med Dent Sci, 2020, 8 (7): 16-15.
Received: 27-Sep-2020 Accepted: 13-Oct-2020
