Research Article - (2022) Volume 10, Issue 10
Diagnostic Markers in Oral Potentially Malignant Disease-Oral Sub Mucous Fibrosis (OSMF)-A Review
Rishika Dhimole, Rahul Bhowate* and Mrunal Meshram
*Correspondence: Dr. Rahul Bhowate, Department of Oral Medicine and Radiology, Sharad Pawar Dental College and Hospital, Datta Meghe Institute of Medical Sciences, (Deemed to be University), Maharashtra, India, Email:
Abstract
In Asian countries, OSF is indeed a cancer risk factor particularly in locations where eating betel nuts is a popular pastime. OSF affects mouth functioning and brought on by aberrantbuild-up of collagen in the connective tissue. Despite the fact that there is no imminent threat associated with a diagnosis, The OSF has a substantial impact on patient well-being. The key etiological factor for OSF is the areca nut. The development of this condition can be linked to enhance collagen synthesis and/or declined collagen breakdown. Chewing betel quid impacts wound healing and fibrotic processes in OSF by promoting fibroblast contraction in the buccal mucosa. TGF-(37,38), NF-B, JNK, P38, and ERK MAPK (39,40) signaling pathways are disrupted in OSF, that may add to areca quid chewing-associated with OSF.
Keywords
Collagen, Fibrotic processes, Buccal mucosa
Introduction
Oral sub mucous fibrosis is an inflammatory disease of the sub mucous tissue that develops over time. It is a morbid condition about oral cavity involving all mucosa in oral cavity and sometimes posterior pharyngeal area causing difficulty in deglutination and speech due to deposition of abnormal fibrous tissue which subsequently leads to hyalinization [1]. Apoptotic changes in the epithelium leads to stiffening of oral mucosae, altered taste sensation due to depapillation of tongue papillae, impaired movement of tongue, difficulty to eat, pain, burning sensation leads to deficiency of vitamin B and C along with altered metabolism of iron and zinc Including the oropharyngeal and esophageal malignancies these patient may suffer from multi systemic disorders like CVS, RS, Liver type 2 diabetes mellitus etc. [2] cause of oral submucous fibrosis is habit of betel nut (areca catechu) chewing. Epithelial proliferation and new vascularization (angiogenesis) results in high risk of malignant transformation, hence it’s a potentially lethal condition. In our country, malignant transformation rate is 7.6%. There are various biomarkers which aids about the diagnosis for oral submucous fibrosis. While several biomarkers for OSF lesions have been identified, and trials with various drug combinations have been conducted, clinicians have continued to adopt conservative treatment approaches that focus primarily on alleviating OSF symptoms. The goal of treatment is to reduce inflammation and improve dental health. In many human tissues, the start and progression of fibrosis are linked to Connective Tissue Growth Factor (CTGF). Arecolinehas been also shown to induce CTGF production in buccal mucosal fibroblasts in a dose and time dependent manner via the reactive oxygen species (ROS), NF–kappa B, JNK, and p38 MAPK pathways. This review attempts to focus on the biomarkers present in the blood as well as in the serum which are specifically related to epithelial proliferation, angiogenesis and apoptosis. Biomarkers related to above events are: AP-1, MAPK, Caspase, P53, NF-KB, Matrix metalloproteinase (MMP2, MMP9), p53, STAT3, EGFR, and m-TORn [3-27].
Literature Review
Risk Factors of OSMF: OSF is caused by immunologic factors like inflammation and autoimmune, as well as carcinogenic reasons such as chewing cigarettes and betel nut, alcohol, hot food intake, epigenetic regulation, and hereditary susceptibility, nutritional factors such as vitamin B, C and iron deficiencies, epigenetic regulation, and hereditary tendency [28-33]. Chili-containing foods irritate the oral mucosa, causing an inflammatory response that can lead to OSF [34]. In terms of nutritional deficit, OSF patients had significantly lower serum-carotene levels [35], iron [36], In a grade dependent manner, vitamin C [37] and zinc; all of these variables are known to impede repair of wound patients have greater serum copper levels [38], which increases the activity of the Lysyl Oxidase (LOX) enzyme, which crosslinks fibrin and elastin.
Increases copper level in the salivahave been linked to an increase in clinical grade [39]. In OSF patients, epigenetic changes were found in the buccal cells'WIF1 [40] and p16 [41] genomes. Hypermethylation of 2 genes mayeven contribute for OSF's malignancy.
Role of epithelial proliferation: Malignant transformation of OSMF preceded by some changes in the epithelium results in dysplasia. According to research, the p63 gene (which is physically and functionally similar to the p53 tumour suppressor gene) is involved in epithelial cell proliferation and differentiation. Surface keratinocytes retain their proliferative potential and hence continue to demonstrate p63 activity in epithelial dysplasia, culminating in architectural changes that eventually lead to oral cancer [3]. As disease progresses it leads to extracellular matrix production (collagen 1, collagen 2 and fibronectin) and results in oral fibrosis. This thusly prompts decrease in the degree of corium vascularity which in turn brings about hypoxia and animates an overexpression of HIF1-α.
Role of Angiogenesis: This is an organic cycle that includes the development of fresh blood vessels. This phenomenon natural part of the body's growth and development, in the healing process of any injury, inflammatory process and malignant condition etc. [2] As fibrosis increases in advanced cases, VEGF (Vascular Endothelial Growth Factor) expression decreases, resulting in reduced and constricted vascular channels, that is, a reduction in the diameter of the vessels [4].
Role of apoptosis: Apoptosis is a process by which a cell actively commits suicide. “Apo” means “separate from” and “ptosis” means “fall from”. It is a programmed cell death, various external or intracellular stimuli can initiate a common cell-death mechanism, can trigger this death programme. Cells in the epithelium are cytotoxic and apoptotic when exposed to areca nuts [42,43].
Discussion
AP-1: Activator protein 1 one of the recorded biomarker controls quality articulation in light of an assortment of upgrades, including cytokines, development chemicals, stress, and bacterial and viral contaminations. Proliferation, differentiation, and apoptosis are just a few of the biological processes that AP1 regulates. The AP1 structure is consist of proteins from the c-Fos, c-Jun, ATF and JDP families [5,6]. The ATF2 (Activating Transcription Factor 2) and AP1 complexes are thought to mediate anticrime control of TGF- β production in many cell types, according to reports in the literature [7-10]. Areca nut-extract consistently increases ATF2 and c-Jun expression [11]. Members of the AP1 family feature a bZIP domain that permits them to homo/ heterodimerize with one another. The binding partner determines the transcription some of the factors regulated the programme [12]. Both ATF-2 and c-Jun was detected inside nuclei of epithelial cells in OSF tissues, but their activity has not been found in normal tissue [11].
Mitogen Activated Protein Kinase (MAPK)
These proteins transmit, enhance, and combine signals from both intracellular and external stimuli (c-Jun Nterminal kinase) a protein belong to MAPK class of protein, was found to be needed for the activation of ATF2 and c-Jun, while TβR-I activity was not essential. Areca nut activation of JNK may be a crucial stage in the beginning of OSF [11].
Epithelial mesenchymal transition in OSMF
The Epithelial to Mesenchymal Transition (EMT) occurs when epithelial cell turns into a cell with mesenchymal characteristics. EMT is a crucial mechanism that happens throughout embryonic development, repair, organogenesis and the start of cancer. The EMT may have a role in cancer invasion and metastasis, according to new evidence. The status of EMT in the malignant development of Oral Sub mucous Fibrosis (OSF) and the inflammatory reaction preceding fibrosis implies a previously unknown involvement of EMT [13]. There are various signaling pathways which regulates EMT in OSMF and OSCC (Figures 1-7) [42].
TGF – β signaling pathway:
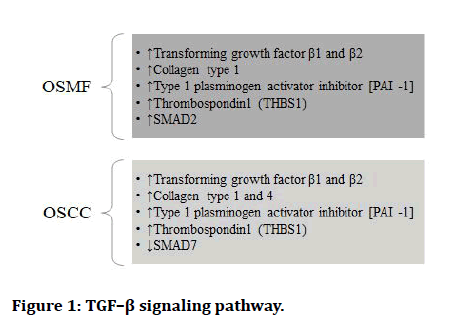
Figure 1: TGF–β signaling pathway.
RTK signaling:
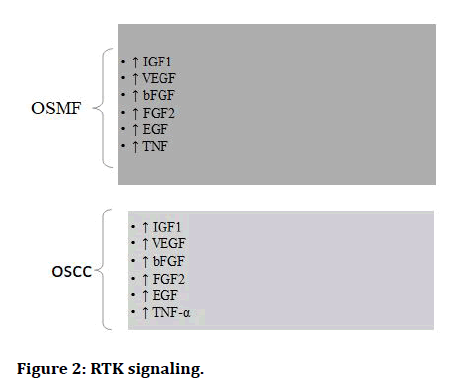
Figure 2: RTK signaling.
Hypoxia signaling pathway:
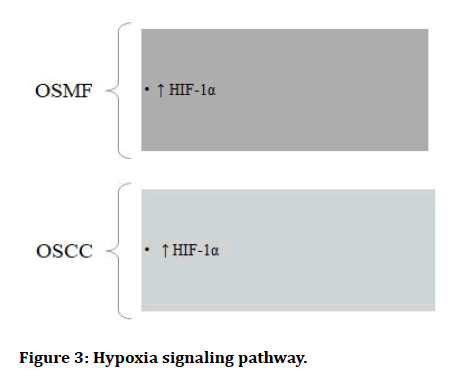
Figure 3: Hypoxia signaling pathway.
Wnt signaling pathway:
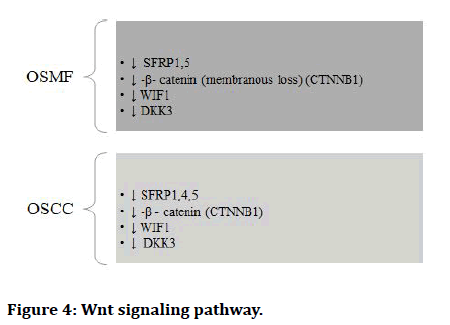
Figure 4: Wnt signaling pathway.
MAPK pathway:
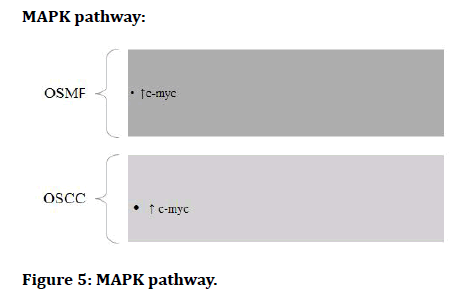
Figure 5: MAPK pathway.
AKT pathway:
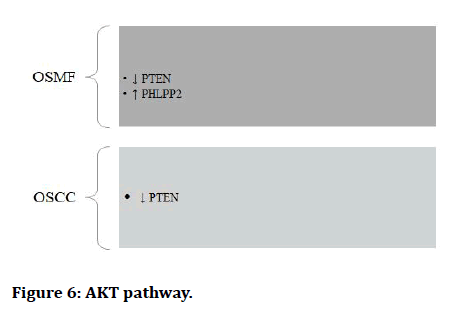
Figure 6: AKT pathway.
Matrix signaling pathway:
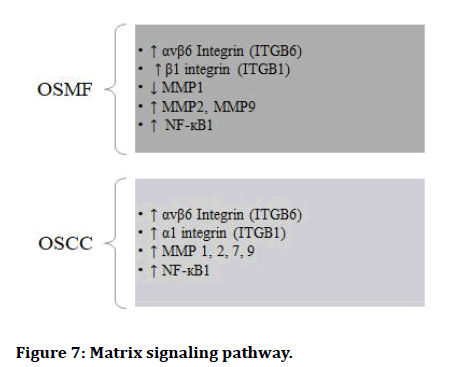
Figure 7: Matrix signaling pathway.
Matrix Metallo Proteinase (MMP): MMPs are calciumdependent zinc-containing endopeptidases, commonly known as matrix metallo peptidases or matrixins [14]. These enzymes can break down a variety of extracellular matrix proteins, as well as processing a variety of biologically active compounds. Cell surface receptor cleavage, the release of apoptotic ligands (FAS ligand), and the deactivation of chemokines and cytokines are all known to be involved [15]. MMPs are thought to influence cell expansion, relocation (grip/scattering), separation, angiogenesis, apoptosis and host protection.
NF-KB: NF-KB regulates DNA replication and cellular longevity. NF-B present in basically is associated with pressure, cytokines, free extremists, substantial metals, UV light, oxidized LDL and bacterial or viral antigen reactions, in addition to other things [2-7]. The transcription factor NF-B is involved in the regulation of the immunological reaction to disease. Malignancy has also been linked to NF-B dysregulation, incendiary and immune system sicknesses, septic shock, viral contamination, and ill-advised immunological turn of events.
Caspaseenzyme: As the Oral Sub Mucous Fibrosis (OSMF) proceeds, epithelial atrophy is supposed to be an aftereffect of stromal fibrosis, hyalinization, decreased vascularity and cellularity, and is referred toas "ischemic atrophy." Caspases 3 may get activated as a result of hypoxia, resulting in a drop in viable cell count.In the majority of instances, the cells in the basal and spinous layers of normal oral mucosa expressed caspase 3 in the nucleus. The expression was found in the nucleus of the upper spinous layer, as well as the superficial layers. The OSMF demonstrated that caspase-3 is primarily expressed in the cytoplasm of basal cells. In several cases of OSMF, nuclear expression was also visible. OSMF cases with dysplasia had either no expression of caspase 3 or a very low % of caspase 3 positive cells [16]. A incitement for either the inherent demise receptor-autonomous pathway or the extraneous passing receptor-subordinate pathway, the two of which require the consecutive activity of cysteine proteases known as caspases, triggers apoptosis [17]. The major executioner of the apoptosis pathway, activated caspase 3, cleaves a vast number of cytoplasmic and nuclear proteins [18]. Caspases 3 analysis of the simple, accurate, and reliable method for detecting apoptosis of its early phases [19]. Caspases 3 were also discovered in the OSMF and OSCC instances. Translocation of activated caspase 3 to the nucleus is indicated by nuclear expression. Caspase 3 is a specific caspase.
Role of Cyclo Oxygenase (COX): OSF has been linked to inflammatory alterations during the course of the illness. Among the most essential mediators of inflammation is prostaglandin, which is produced by a variety of enzymes including Cyclo-Oxygenase (COX). According to research, the enzyme's expression is higher in intermediate fibrosis and disappears in advanced fibrosis. Collagen accumulation in buccal mucosal fibroblasts has already been discovered as a result of alkaloid (arecoline) exposure.
Fibroblast Growth Factor (FGF): FGFs, which frequently interact additional growth elements in a synergistic manner, may influence extracellular matrix accumulation. BFGF biological action in fibroblasts and the encompassing lattice its contribution as a middle person in the pathogenic course of OSF is specifically noteworthy. In the early phases of fibrosis, as well as cytokine networks potentially open up new therapeutic avenues and improve OSF treatment alternatives. In OSF patients, expression of BFGF in endothelial cells and fibroblasts was observed to be higher, as BFGF promotes leukocyte recruitment during inflammation by increasing expression of endothelial adhesion molecules [21]. Research has also shown that IL-1 and BFGF are produced by endothelial cells, which might be associated with the pathogenesis of fibrosis through modified endothelial cell–fibroblast correspondence. It has been recommended that pole cells are a significant wellspring of heparin in the beginning phases of OSF, and that they might fill in as a critical wellspring of heparin restricting development factor, the BFGF [23]. A decrease in mesenchymal cell concentration and aberrant extracellular cytokine deposition could explain the anomalous mesenchymal distribution of BFGF in OSF [24]. Another possibility for the observed aberration in OSF is a malfunction in either ligand synthesis or cytokine deposition on the cell surface. As a result, a failure in BFGF extracellular binding could be caused by a distinct group of BFGF binding molecules, such as heparin-like glycosaminoglycans. Using cultured oral fibroblasts, the direct effects of BFGF-1 and TGF-1 on fibroblast proliferation and collagen production have revealed differentiation and extracellular matrix build up. TGFB has been demonstrated to be auto inductive& anabolic, whereas BFGF was auto repressive and catabolic, thus reflecting a portion of the feedback system governing stromal development. When BFGF and TGF were combined, however, anabolic properties won out. In TMJ disc cells, however BFGF was viewed as the best development factor in helping expansion, glycosaminoglycan amalgamation and collagen combination [25].
Malignant transformation of OSMF: As OSMF is a potentially malignant condition it can further land up into malignancy if the etiological factors persist. Paymaster first discovered the precancerous aspect of OSF in 1956, once he observed that one-third of the individuals had sluggish SCC along the disease [26]. According pindborg [1972] some criteria are there for differentiating the malignant transformation of OSMF (Figure 8) [27].
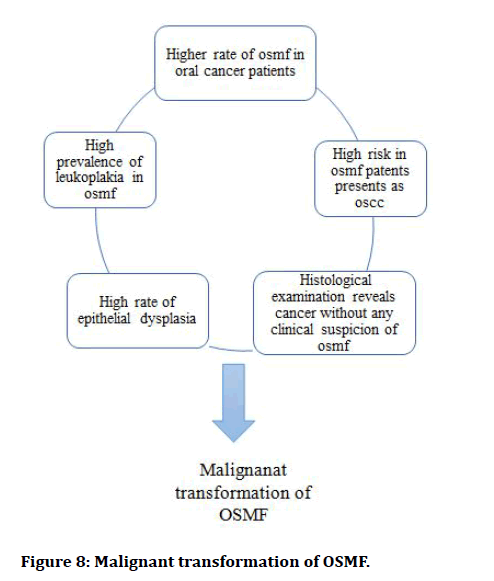
Figure 8: Malignant transformation of OSMF.
The rate of malignant transformation of OSMF in India is 7.6%. The authors believe that extensive fibrosis and decreased vascularity of the corium, together with, adjusted cytokine movement, establish a novel climate where cancer-causing agents from tobacco and are coline can follow up on epithelial layer. It's conceivable that cancer-causing agents from areca nut gather over the long haul, they can work for a longer duration on or directly underneath the epithelium, allowing them to act for a longer time before diffusing into deeper tissues. Carcinogens may not be absorbed as quickly into the systemic circulation if there is less vascularity. The fact that carcinomas, rather than sarcomas, are frequently reported cases of OSF and the malignant change it undergoes remains intriguing in pathophysiology of the disease and its eventual malignant transformation.
EGFR: The level of EGFR articulation in a premalignant injury seems, by all accounts, to be a touchy marker of neoplastic potential. This shows that EGFR could be utilized as a biomarker to distinguish high hazard populaces and guide prophylactic therapy with chemo preventive meds or careful mediation to forestall disease movement. The Epidermal development Factor Receptor (EGFR) is a tyrosine kinase that has a place with the ERBB group of receptors. The EGFR gene, which codes for a 170-kDa transmembrane glycoprotein, is found on chromosome 7p11.2. Changes in EGFR function have been associated to oncogenic transformation, autonomous cell proliferation, invasion, angiogenesis, and metastasis development in numerous malignancies, and are important tumour features [44]. Oral cancerous keratinocytes have 5 to 50 times more EGFR than normal keratinocytes [45]. Many types of cancer cells have abnormally high quantities of it on their surfaces; therefore these cells may divide excessively in the presence of it. EGF is a type of epidermal growth factor. It's also known as HER1 and ERBB1 [46].
STAT-3: STAT3, a transcriptional regulator in the STAT family, is activated through tyrosine phosphorylation via upstream receptors including epidermal growth factor, PDGF, and others. Interleukin-6, for example, is a kind of cytokine. STAT 3 is a significant mediator by their influence on carcinogenesis and its implications apoptosis, cell proliferation, and angiogenesis are some of the parameters studied and evasion of the immune system [47].
Akt/mTOR: The Akt/mTOR pathway can be activated to start cyclin D1 translation without impacting its transcription or stability. As a result, studies have observed at the phosphorylation of associated proteins to see how are coline affected following pathway. Carcinogenes is aided by protein kinase B (AKT)/ mammalian Target of Rapamycin (MTOR) pathway. The AKT protein is essential for normal and cancer cell proliferation and survival, and its downstream protein, MTOR, is equally important in cellular functions. Oral squamous cell carcinoma has been found to have a broad involvement of the AKT/MTOR pathway (OSCC). Multiplication, endurance, angiogenesis, attack, metastasis, autophagy, and epithelial-to-mesenchymal progress are a couple of the signs of malignancy that this hub controls (EMT). Circadian signalling, chemo resistance, and radio resistance are all linked to activate AKT/MTOR signalling. Cell multiplication; endurance, attack, angiogenesis, movement, protein blend and glucose digestion are totally helped by this pathway [48].
Through the AKT/mTOR aberration-induced amplified expression of HIF-1, the immune regulatory protein B7- H3, sometimes called CD276, OSCC cells have their aerobic metabolism increased, resulting in increased proliferation of OSCC cells [49].
In both normal and malignant tissues, this pathway is crucial for controlling blood vessel development. The release of VEGF, b-FGF and interleukins (IL-8) characterizes angiogenesis. Malignant cells produce interleukin-8 (IL-8) [50,51]. Angiogenesis is the process of blood vessels forming and delivering growth factors to tumours. Cells with abnormal MTOR, on the other hand, another changed signalling system helps signalling adapt and survive in nutrient depleted and hypoxic circumstances.
P53: It is involved in the cell cycle in the following ways: The p53 protein is the mastermind behind a wellcoordinated system for detecting and controlling cellular harm. The p53 protein assists in the selection between repair and cell death as soon as the protein network detects the damage. Apoptosis is the name for this process. TSN’s (Tanshinone’s) main cellular target in OSF is the p53 pathway p53, quite possibly the most often changed and erased cancer suppressor in an assortment of growth types, has regularly been accounted for to be down regulated or erased in oral squamous cell carcinoma, suggesting that different components in OSF, the precancerous injury likely liable for p53 down regulation, are working. In OSF, p53 expression was substantially lower than in normal mucous tissues [52].
Conclusion
The frequency of OSF and the rate at which it transforms into cancer varies by country. The easiest way to avoid OSF and probable malignancy is to stop chewing betel nuts. The areca nut is a "category one human carcinogen," according to the World Health Organization. The areca nut's carcinogenicity has been demonstrated by the connection of OSMF with OSCC [55,56]. The oral mucosa's flexibility and mouth opening distance must be improved as part of the treatment. There is a change in the micro biome, which could be used as a marker for OSMF malignancy [57]. This guarantees that patients have typical oral capacities like talking and eating, so improving their quality of life and ensuring proper nutritional intake. Clinical trials of high quality are required to aid doctors in the development and application of molecular biomarkers and the formulation of standard treatment guidelines for OSF. Upstream and downstream signalling processes involving these markers may be altered in the OSCC-OSF group, requiring further investigation. This can be attributed to the areca nut's and its constituents' particular carcinogenicity [53]. Because it has been closely connected with the of these patients, socioeconomic aspects should be incorporated in the holistic care of OSF [54].
References
- Cox SC, Walker DM. Oral submucous fibrosis. A review. Aust Dent J 1996; 41:294–299.
- Shen YW, Shih YH, Fuh LJ, et al. Oral Submucous Fibrosis: A Review on Biomarkers, Pathogenic Mechanisms, and Treatments. Int J Mol Sci 2020; 21:7231.
- Matsubara R, Kawano S, Kiyosue T, et al. Increased ΔNp63 expression is predictive of malignant transformation in oral epithelial dysplasia and poor prognosis in oral squamous cell carcinoma. Int J Oncol 2011; 39:1391-1399.
- Sharma E, Tyagi N, Gupta V, et al. Role of angiogenesis in oral submucous fibrosis using vascular endothelial growth factor and CD34: An immunohistochemical study. Indian J Dent Res 2019; 30:755-762.
- Hess J, Angel P, Schorpp-Kistner M. "AP-1 subunits: quarrel and harmony among siblings". J Cell Sci 2004; 117:5965–7593.
- Lee W, Haslinger A, Karin M, et al. Activation of transcription by two factors that bind promoter and enhancer sequences of the human metallothionein gene and SV40. Nature 1987; 325:368–372.
- Kim SJ. Autoinduction of transforming growth factor beta 1 is mediated by the AP-1 complex. Mol Cell Biol 1990; 10:1492–1497.
- Kim SJ. Retinoblastoma gene product activates expression of the human TGF-beta 2 gene through transcription factor ATF-2. Nat Med 19923; 358:331–334.
- Presser LD, McRae S, Waris G. Activation of TGF-β1 promoter by hepatitis C virus-induced AP-1 and Sp1: role of TGF-β1 in hepatic stellate cell activation and invasion. Plos One 2013; 8:e56367.
- Weigert C. AP-1 proteins mediate hyperglycemia-induced activation of the human TGF-β1 promoter in mesangial cells. J Am Soc Nephrol 2000; 11:2007–2016.
- Pant I, Rao SG, Kondaiah P. Role of areca nut induced JNK/ATF2/Jun axis in the activation of TGF-β pathway in precancerous Oral Submucous Fibrosis. Sci Rep 2016; 6:34314.
- Dam HV, Castellazzi M. Distinct roles of Jun: Fos and Jun: ATF dimers in oncogenesis. Oncogene 2001; 20:2453–2464.
- Shetty SS, Sharma M, Fonseca FP, et al. Signaling pathways promoting epithelial mesenchymal transition in oral submucous fibrosis and oral squamous cell carcinoma. Jpn Dent Sci Rev 2020; 56:97–108.
- Verma RP, Hansch C. Matrix metalloproteinases (MMPs): chemical-biological functions and (Q)SARs. Bioorg Med Chem 2007; 15:2223–2268.
- Van Lint P, Libert. "Chemokine and cytokine processing by matrix metalloproteinases and its effect on leukocyte migration and inflammation". J Leukoc Biol 2007; 82:1375–1381.
- Veeravarmal V, Austin RD, Siddavaram N, et al. Caspase-3 expression in normal oral epithelium, oral submucous fibrosis and oral squamous cell carcinoma. J Oral Maxillofac Pathol 2016; 20:445-452.
- Singh TD, Lee HW, Lee SW, et al. Non-invasive imaging of apoptosis induced by adenovirus‑mediated cancer gene therapy using a caspase‑3 biosensor in living subjects. Mol Imaging 2014; 13.
- Lu X, Arbiser JL, West J, et al. Tumor necrosis factor‑related apoptosis‑inducing ligand can induce apoptosis in subsets of premalignant cells. Am J Pathol 2004; 165:1613‑1620.
- Duan WR, Garner DS, Williams SD, et al. Comparison of immunohistochemistry for activated caspase‑3 and cleaved cytokeratin 18 with the TUNEL method for quantification of apoptosis in histological sections of PC‑3 subcutaneous xenografts. J Pathol 2003; 199:221‑228.
- Jian X, Jian Y, Wu X, et al. Oral submucous fibrosis transforming into squamous cell carcinoma: a prospective study over 31 years in mainland China. Clin Oral Investig 2021; 25:2249-2256.
- Zittermann SI, Issekutz AC. Endothelial growth factors VEGF and bFGF differentially enhance monocyte and neutrophil recruitment to inflammation. J Leukoc Biol 2006; 80:247-257.
- Wojas-Pelc A, Lipko-Godlewska S. Patogenezatwardzinyskornej-przegladpismiennictwa (Pathogenesis of skin scleroderma-literature review). Przegladlekarski 2005; 62:310–313.
- Blumenthal R, Hansen H, Goldenberg D. In vitro and in vivo anticancer efficacy of unconjugated humanised anti-CEA monoclonal antibodies. Br J Cancer 2008; 99:837–838.
- Yoon JC, Puigserver P, Chen G, et al. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature 2001; 413:131–138.
- Detamore MS, Orfanos JG, Almarza AJ, et al. Quantitative analysis and comparative regional investigation of the extracellular matrix of the porcine temporomandibular joint disc. Matrix Biol 2005; 24:45–57.
- Paymaster JC. Cancer of the buccal mucosa. A clinical study of 650 cases in Indian patients. Cancer 1956; 9:431-435.
- Pindborg JJ, Murti PR, Bhonsle RB, et al. Oral submucous fibrosis as a precancerous condition. Scand J Dent Res 1984.
- Passi D, Bhanot P, Kacker D, et al. Oral submucous fibrosis: Newer proposed classification with critical updates in pathogenesis and management strategies. Natl J Maxillofac Surg 2017; 8:89–94.
- Balakrishnan C, Aswath N. Estimation of serum, salivary immunoglobulin G, immunoglobulin A levels and total protein, hemoglobin in smokeless tobacco chewers and oral submucous fibrosis patients. Contemp Clin Dent 2015; 6:S157-S162.
- Arakeri G, Rai KK, Hunasgi S, et al. Oral submucous fibrosis: An update on current theories of pathogenesis. J Oral Pathol Med 2017; 46:406-412.
- Guruprasad R, Nair PP, Singh M, et al. Serum vitamin c and iron levels in oral submucous fibrosis. Indian J Dent 2014; 5:81-85.
- Wang YP, Wu YC, Cheng SJ, et al. High frequencies of vitamin B12 and folic acid deficiencies and gastric parietal cell antibody positivity in oral submucous fibrosis patients. J Formos Med Assoc 2015; 114:813-819.
- Teh MT, Tilakaratne WM, Chaplin T, et al. Fingerprinting genomic instability in oral submucous fibrosis. J Oral Pathol Med2008; 37:430-436.
- Seedat HA, van Wyk CW. Submucous fibrosis in non-betel nut chewing subjects. J Biol Buccale 1988; 16:3-6.
- Raina C, Raizada RM, Chaturvedi VN, et al. Clinical profile and serum beta-carotene levels in oral submucous fibrosis. Indian J Otolaryngol Head Neck Surg 2005; 57:191–195.
- Thakur M, Guttikonda VR. Estimation of hemoglobin, serum iron, total iron-binding capacity and serum ferritin levels in oral submucous fibrosis: A clinicopathological study. J Oral Maxillofac Pathol 2017; 21:30-35.
- Guruprasad R, Nair PP, Singh M, et al. Serum vitamin c and iron levels in oral submucous fibrosis. Indian J Dent 2014; 5:81-85.
- Sachdev PK, Freeland-Graves J, Beretvas SN, et al. Zinc, Copper, and Iron in Oral Submucous Fibrosis: A Meta-Analysis. Int J Dent 2018; 3472087.
- Vallet SD, Ricard-Blum S. Lysyl oxidases: From enzyme activity to extracellular matrix cross-links. Essays Biochem 2019; 63:349-364.
- Zhou S, Chen L, Mashrah M, et al. Expression and promoter methylation of Wnt inhibitory factor-1 in the development of oral submucous fibrosis. Oncol Rep 2015; 34:2636-2642.
- Kaliyaperumal S, Sankarapandian S. Evaluation of p16 hypermethylation in oral submucous fibrosis: A quantitative and comparative analysis in buccal cells and saliva using real-time methylation-specific polymerase chain reaction. South Asian J Cancer 2016; 5:73-79.
- Smitha Sammith Shetty, Mohit Sharma, Felipe Paiva Fonseca, et al. Signaling pathways promoting epithelial mesenchymal transition in oral submucous fibrosis and oral squamous cell carcinoma. Jpn Dent Sci Rev 2020; 56:97-108.
- Li M, Gao F, Zhou ZS, et al. Arecoline inhibits epithelial cell viability by upregulating the apoptosis pathway: Implication for oral submucous fibrosis. Oncol Rep 2014; 31:2422-2428.
- Srinivasan M, Jewell SD. Quantitative analysis of PCNA, cmyc, EGFR and TGF-α in oral submucous fibrosis-an immunohistochemical study. Oral Oncol 2001; 37:461-467.
- Cawson RA, Odell EW. Cawson’s Essentials of Oral Pathology and Oral Medicine, 8th edition. London: Elsevier; 2008; 261-276.
- Chiang WF, Liu SY, Yen CY. Association of Epidermal Growth Factor Receptor (EGFR) gene copy number amplification with neck lymph node metastasis in areca-associated oral carcinomas. Oral Oncol 2008; 44:270-276.
- Ingle E. Turmeric in the management of oral submucous fibrosis-A systematic review and meta-analysis. Int J Health Sci (Qassim) 2020; 14:41-46.
- Liu P, Cheng H, Roberts TM, et al. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Reviews Drug Discov 2009; 8:627–644.
- Li Z, Liu J, Que L, et al. The immunoregulatory protein B7-H3 promotes aerobic glycolysis in oral squamous carcinoma via PI3K/Akt/mTOR pathway. J Cancer 2019; 10:5770–5784.
- Shanmugam MK, Warrier S, Kumar AP, et al. Potential Role of Natural Compounds as Anti-Angiogenic Agents in Cancer. Curr Vasc Pharmacol 2017; 15:503-519.
- Sethi G, Chatterjee S, Rajendran P, et al. Inhibition of STAT3 dimerization and acetylation by garcinol suppresses the growth of human hepatocellular carcinoma in vitro and in vivo. Mol Cancer 2014; 13:66.
- Zheng L, Guan ZJ, Pan WT, et al. Tanshinone Suppresses Arecoline-Induced Epithelial-Mesenchymal Transition in Oral Submucous Fibrosis by Epigenetically Reactivating the p53 Pathway. Oncol Res 2018; 26:483-494.
- Hande AH, Chaudhary MS, Gawande MN, et al. Oral submucous fibrosis: An enigmatic morpho-insight. J Cancer Res Ther 2019; 15:463.
- Gadbail AR, Korde S, Chaudhary MS, et al. Ki67, CD105, and α-SMA expression supports biological distinctness of oral squamous cell carcinoma arising in the background of oral submucous fibrosis. Asian Pac J Cancer Prev 2020; 21:2067.
- Gondivkar SM, Bhowate RR, Gadbail AR, et al. Impact of socioeconomic inequalities on quality of life in oral submucous fibrosis patients. Future Oncol 2019; 15:875-883.
- .Hande AH, Chaudhary MS, Gawande MN, et al. Oral submucous fibrosis: An enigmatic morpho-insight. J Cancer Res Ther 2019; 15:463.
- Hande AH, Sonone A, Porwar R, et al. Evaluation of Oral Microbial Flora in Saliva of Patients of Oral Submucous Fibrosis. J Evol Med Dent Sci 2020; 9:409-412.
Author Info
Rishika Dhimole, Rahul Bhowate* and Mrunal Meshram
Department of Oral Medicine and Radiology, Sharad Pawar Dental College and Hospital, Datta Meghe Institute of Medical Sciences, (Deemed to be University), Maharashtra, IndiaCitation: Rishika Dhimole, Rahul Bhowate, Mrunal Meshram, Diagnostic Markers in Oral Potentially Malignant Disease- Oral Submucous Fibrosis (OSMF)-A Review, J Res Med Dent Sci, 2022, 10 (10): 133-140.
Received: 11-Aug-2022, Manuscript No. JRMDS-22-47349; , Pre QC No. JRMDS-22-47349(PQ); Editor assigned: 16-Aug-2022, Pre QC No. JRMDS-22-47349(PQ); Reviewed: 30-Aug-2022, QC No. JRMDS-22-47349; Revised: 12-Oct-2022, Manuscript No. JRMDS-22-47349(R); Published: 20-Oct-2022
