Research - (2021) Volume 9, Issue 4
Development, Characterization and Evaluation of Nano Formulation of Abrus Precatorius Root Extract for Anti Inflammatory Activity
Sneha Nawale*, Sindhu Bhargavi M, Prasanna Laxmi K and Ganga Raju M
*Correspondence: Sneha Nawale, Department of Pharmacology, Gokaraju Rangaraju College of Pharmacy, India, Email:
Abstract
The aim of the present study was to develop nano formulation of Abrus precatorius root extract (NE-AP) and characterize for particle size, poly dispersed index (PDI), entrapment efficiency, zeta potential, SEM, XRD and differential scanning microscopy (DSC). NE-AP was assessed for its anti-inflammatory potential with carrageenan induced rat paw edema, formalin induced rat paw edema and cotton pellet granuloma in rats. Average particle size of NE-AP was found 35 nm, PDI 60%, entrapment efficiency 46%, zeta potential -23mV. XRD showed the characteristic peaks at 32, 41 and 67 θ. SEM revealed the spherical shape and smooth surface of particle, and in DSC melting endotherm was observed at 329.37º C. NE-AP (50 mg/kg bd.wt and 100 mg/kg bd.wt) showed significant antiinflammatory activity with carrageenan induced inflammation (p˂0.01, p˂0.05), formalin induced inflammation (p˂0.01, p˂0.05), and cotton pellet granuloma (p˂0.01, p˂0.05) when results were compared with standard and disease control animals. Nanoemulsion of Abrus precatorius [NE-AP] showed prominent anti-inflammatory activity so can be used for management of anti-inflammatory activity with improved permeability.
Keywords
Anti-inflammatory activity, Abrus precatorius, Nano emulsion, Silver nitrate
Introduction
Inflammation is a physical response acts through various mechanism and protects against injury, infection, and stress. Oxidative stress that occurs in inflammation causes tumor progression, risk of atherosclerosis and coronary heart disease, lesions of Alzheimer’s disease, and promote insulin resistance and diabetes [1-3]. As inflammation is a key cause, treatment of the inflammatory condition may be an effective therapeutic approach. Important medicines for treating the inflammation belong to NSAID and steroids category have severe adverse effects, so there is need to search for alternative from herbals with less adverse effect [4,5]. Therapeutic effects of herbal medicine is due to active secondary metabolites [6] conversely most of these active secondary components have less solubility leading to low permeability and bioavailability, improved systemic clearance [7], so required unnecessarily high dose administration for particular action [8]. Application of nanotechnology to herbal drugs may lead to the development of nano herbal products, which may enhance solubility, bioavailability, pharmacological action and sustained delivery, also protect from toxicity, physical and chemical degradation [9]. At nanoscale scale (1–100 nm), materials exhibit some unusual new properties which can’t be defined with classical laws of physics [10,11]. These properties have paying attention of researchers from every science field including biology and medicine [10]. Amongst available nanoparticles, silver nanoparticles are non-toxic to human health [12] and possess anti-inflammatory [5], antiviral [13], and antibacterial [14] activities, so being an excellent choice in the medical field [15]. A precatorius is a woody twinning plant with various pharmacological actions as anti-diabetic [16], anti-oxidative [17], neuroprotective, antiviral [18], neuromuscular, anti-convulsant, antiepileptic, immune-modulating, abortifacient, [11,19] anti-implantation [20,21], anti-helmintic, anti-depression [22], anti-inflammatory [22,23]. Abrus precatorius root extract reported to contain biologically active secondary metabolites as abrol, abrasine, precasine and precol [24,25], abraline, abricin, abrusgenic acid, anthocyanins, campesterol, cycloartenol, delphinidin, gallicacid, trigonelline, hypaphorine [26,27] P coumaroylgalloyl glucodelphinidin, delphinidin, picatorine, precatorine [28], isoflavonoids and quinones-abruquinones A, B, C, D, E, F [29], O, G, abruslactone a, [30,31], Triterpenoids, saponins [32] glycyrrhizin [33] and oleanolic acid and abrusosides A, B, C, D 33 and new flavonoid 7,5-dihydroxy-6,49-dimethoxy isoflavone 7-O-b-D- galactopyranoside [34]. The present study was undertaken to investigate the antiinflammatory activity of Nano formulation prepared from Abrus precatorius (NE-AP) root extract.
Materials and Methods
Plant collection, identification and extraction of Abrus precatorius
Abrus precatorius roots were collected from Vikarabad, Hyderabad, Telangana. Crude plant material was identified and authenticated by Dr. P. Suresh Babu, Botanist, (Voucher specimen no., APS-6), Government Degree College, Kukatpally Hyderabad (T.S.), and India. Abrus precatorius crude plant material was cleaned, shade dried and coarsely powdered. Powdered crude material was subjected to soxhlation with methanol and crude methanol extract was dried and stored for further use.
Preparations of nano emulsion of Abrus precatorius root extract (NE-AP)
Abrus precatorius root extract was added in Tween 80 (surfactant; 2 wt% prepared in deionised water). Aqueous solution AgNo3 (2.13Mm, 0.362g) was added drop wise Abrus precatorius extract solution with sonication (Probe sonicator-HD 2070, Source - Bandelin Sonopuls, Germany). The reaction mixture was subjected to continuous stirring at room temperature for 2 h to form dark brown emulsion [NE-AP] contained silver particle [35].
Characterization of Nano emulsion Abrus precatorius root extract (NE-AP)
Particle Size Distribution, poly dispersity index (PDI) and Zeta potential
The NE-AP was analyzed for its particle size, PDI (particle homogeneity in the dispersion), and zeta potential using Zetasizer (Nano ZS, Malvern Instruments, Malvern, UK). The particle size was measured by Particle size analyser (Nanotrac wave, Model: - W-3275, Microtrac, USA) using dynamic light scattering technique.
Entrapment efficiency
The entrapment efficiency of NE-AP was determined by sonicating NE-AP at 20,000 RPM for 20 minutes. Aliquots of above solutions (0.1mL, 0.2mL, 0.3mL, 0.4mL and 0.5mL) were taken and diluted upto 10 mL. Resultant concentrations were centrifuged at 1500 RPM for 20 minutes. The aliquot of supernatant was measured by UV/visible spectrophotometry at 418 nm [36]. Entrapment efficiency (%) was calculated using this formula: % Entrapment efficiency = (Total amount of drug−free drug in supernatant)/Total amount of drug×100.
Morphological Analysis by Scanning Electron Microscopy (SEM) [37]
Scanning electron microscopy was performed at high magnifications, generates high-resolution images, and precisely measures very small features and objects. The surface morphological characteristics and particle size of NE-AP were carried out using a scanning electron microscope (Hitachi-S 3400N) at an acceleration voltage of 10.0 kV. SEM analysis was done in Central Analytical facility-University college of Technology, Osmania University, Hyderabad, Telangana, India.
Zeta potential
The zeta potential of the Nano particles was determined with a Zetasizer NanoZS instrument from Malvern Co. Ltd., UK equipped with a He-Ne laser. Zeta potential values are determined from the electrophoretic mobility of the particles with the laser Doppler technique. The samples were dispersed in milli Q water with a pH of 5.7
Differential scanning calorimetry (DSC) [38]
DSC is a productive technique used for evaluation of thermal properties of NE-AP. NE-AP was subjected to heating rate of 10°C/min from 40°C to 300°C; the heat absorbed or evolved was recorded as exotherm or endotherm. DSC analysis was done in Central Analytical facility- University college of Technology, Osmania University, Hyderabad, Telanagana, India.
UV–vis spectrophotometry
UV/visible absorption spectra of NE-AP was measured with UV/visible spectrophotometer Cary300 (Agilent).
X-ray diffraction (XRD)
XRD analysis of NE-AP was performed using PAN anlytical's X-ray diffractometer equipped with X'Celerator high speed detector. XRD analysis was done in Central Analytical facility-University college of Technology, Osmania University, Hyderabad, Telanagana, India.
Animals
Wistar rats weighing about 160-180 g were procured from Jeeva Life sciences, Uppal, Hyderabad for present experimental study and under standard conditions of temperature (23 ± 20c) humidity and dark-light cycle. The data protocol was approved by the IAEC (Institutional Animal Ethical Committee Reg. No.1175/PO/ Re/S/08/CPCSEA) of CPCSEA (Committee for control and supervision of experimentation on animals).
Anti-inflammatory activity
Carrageenan-induced rat paw edema
The anti-inflammatory activity of Nano emulsion Abrus precatorius root extract (NE-AP) was evaluated by carrageenan-induced rat paw oedema method [39]. Wistar albino rats (160- 180g) were used in the present rat paw oedema assay. The rats were divided into 5 groups of 6 animals each. Group I animals were given normal saline, Group II animals were treated with carragenan (1%w/v) in saline in the subplanter region of the right hindpaw, Group III and Group IV animlas were treated with NE-AP at 50 mg/ kg bd.wt p.o. &100 mg/kg bd.wt. p.o., Group V animals were administered Indomethacin (10 mg/kg, bd. wt., p.o.) considered as standard.
Carrageenan suspension (0.1 mL of 1% w/v s.c.) was injected into plantar surface of the right hind paw after 1 h and paw volume was measured using the plethysmometer at 60, 120, 180 and 240 min post carrageenan injection.
The anti-inflammatory activity was calculated by using the relation
% inhibition of edema = T−T0T×100
T: Thickness of paw in control group; T0: Thickness of paw edema in the test compound treated group.
Formalin –induced rat paw edema
NE-AP was evaluated for formalin-induced rat paw edema with method described by Winter et al. [39] Wistar albino rats (160-180g) were used in the present rat paw oedema assay. The rats were divided into 5 groups of 6 animals each. Group I animals were given normal saline, Group II animals were treated with formalin (2 % w/v/ kg bd. wt.) in saline in the subplanter region of the right hindpaw, Group III and Group IV animals were treated with NE-AP at 50 mg/ kg bd.wt p.o. &100 mg/kg bd.wt. p.o., Group V animals were administered Indomethacin (10 mg/kg,bd. wt., p.o.) considered as standard. Formalin (2 % w/v s.c.) was injected into plantar surface of the right hind paw after 1 h and paw volume was measured using the plethysmometer at 60, 120, 180 and 240 min post formalin injection.
Effects in cotton pellet granuloma
Wistar rats were divided into four groups of six animals each. After shaving the groin region, experimental animals were anaesthetized with light ether anesthesia. Sterile pre-weighed cotton pellets (30 ± 1 mg) were implanted in the groin region of animal through a single needle incision. Group I animals were given normal saline, Group II and Group III animals were treated with NE-AP at 50 mg/kg bd.wt p.o. &100 mg/kg bd.wt. p.o. respectively and Group IV animals were treated with indomethacin10mg/ kg bd.wt. for seven consecutive days from the day of cotton pellet implantation. Animals were anaesthetized on the 8th day; the cotton pellets were removed surgically and made free from extraneous tissues. After 24h of incubation at 37 0C, pellets were dried in an oven at 60 0C to constant weight. The increase in the dry weight of the pellets was considered as a measure of granuloma formation [40,41].
Statistical analysis
The results were expressed as mean ± SEM. Statistical data analysis was done by one way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test. Graph Pad Prism version 8.0 for Windows was used for statistical calculation.
Results and Discussion
The present research work was aimed to formulate Abrus precatorius root extract nanoformulation (NE-AP) for the evaluation of anti inflammatory activity. Abrus precatorius roots were extracted by soxhlation with methanol and methanolic extract was used in formulation. Nanoemulsion of Abrus precatorius was formulated to increase biological activity with increase in solubility and permeability as secondary metabolites are associated with slow and insufficient absorption with inconsistent bioavailability. Formulated nanofoemulation (NE-AP) was characterised for its particle size, particle size distribution, zeta potential and morphological features with SEM. Their SEM images revealed the particle size of NE-AP within the accepted range of nanoparticles. The average Particle size, PDI and zeta potential for NE-AP nanoformulation were found to be 0.035 nm, 60 %; 0.107, and -23mV respectively (Figures 1 and 2).
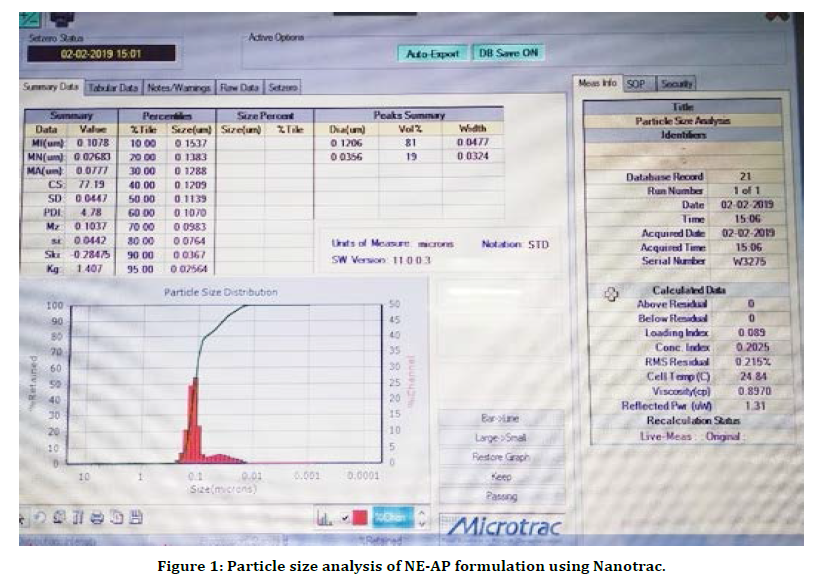
Figure 1. Particle size analysis of NE-AP formulation using Nanotrac.
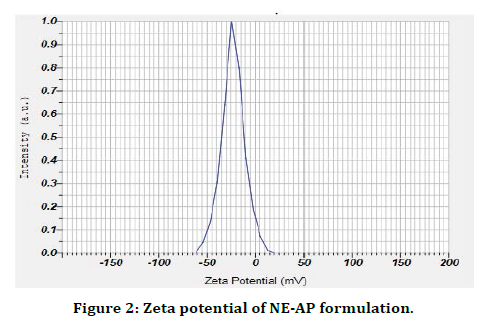
Figure 2. Zeta potential of NE-AP formulation.
The poly dispersity index values represent the average uniformity of a particle solution. Large PDI values ranged 0.107 ± 0.04 (60 %) represent particle size distribution as well as the stability of the nano formulation. Zeta potential shows the charge on the particle surface which indicates the physical stability of dispersed systems. Zeta potential of NE-AP was -23mV indicating that the nanoparticles have a anionic charge (Figure 2).Nanonisation of herbal extracts can lead towards increase in dissolution velocity, wetting, particle surface area and saturation solubility. This may in turn bring about more bioavailability due to enhanced in vivo release as only solubilised particles can be absorbed through lipophilic cellular membranes [42]. These nanostructure systems might be able to potentiate required action of herbal extracts, reducing the necessary dosage, side effects and improved biological activity. Scanning electron microscopy was performed to study surface morphology of NE-AP, which revealed their smooth texture, spherical shape and smooth topology (Figure 3).
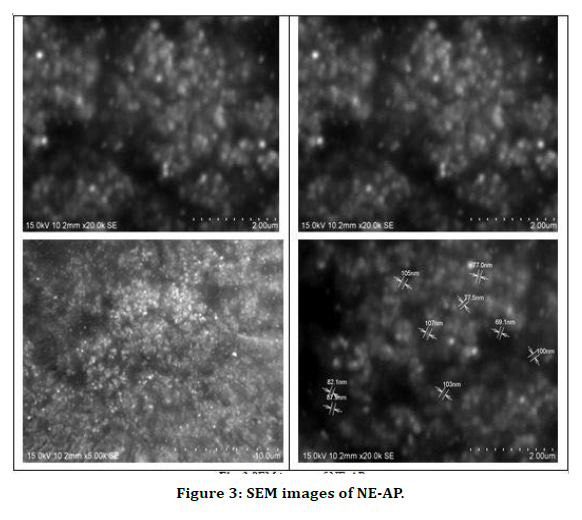
Figure 3. SEM images of NE-AP.
DSC is a productive technique used for evaluation the thermal properties of NE-AP formulation. The thermogram of NE-AP (Figure 4) exhibited endotherm at 330.1°C and 336.8°C. This is may be due to the conversion of secondary metabolites from crystalline to amorphous form. The percentage drug entrapment efficiency was calculated for NE-AP and was found to be 46%. Further XRD pattern of NE-AP was done; clearly indicated diffraction peaks appeared at 32°, 41° and 67° (Figure 5).
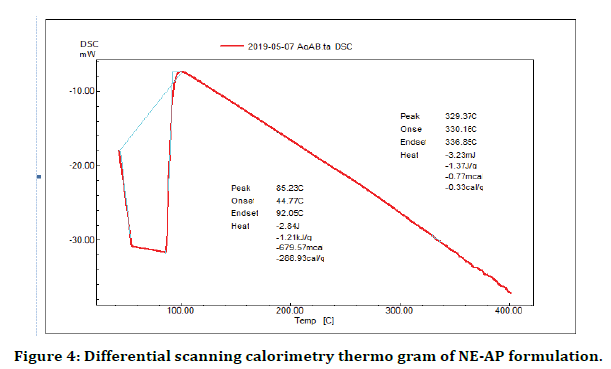
Figure 4. Differential scanning calorimetry thermo gram of NE-AP formulation.
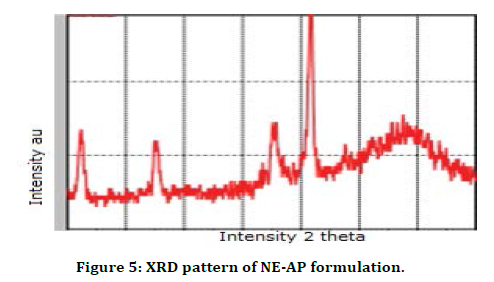
Figure 5. XRD pattern of NE-AP formulation.
In acute carrageenan and formalin induced inflammation model significant decrease in paw volume was observed in NE-AP treated animals at dose 50 and 100 mg/kg bd.wt. When results were compared with disease control (P<0.01, P<0.05) and standard group(P<0.01, P<0.05) treated with indomethacin at dose 10 mg/kg (Tables 1 and 2).
| Groups/Treatment | Change in paw valume (mL) at | Percentage inhibition at 4 h | |||
|---|---|---|---|---|---|
| 1h | 2h | 3h | 4h | ||
| Disease control | 2.65 ± 0.01 | 2.51 ± 0.01 | 2.33 ± 0.013 | 2.17 ± 0.05** | - |
| NE-AP (50mg/kg bd.wt) | 2.23 ± 0.011**a | 2.04 ± 0.01**a | 1.811 ± 0.01**a | 1.546 ± 0.011**a | 28.57 |
| NE-AP (100mg/kg bd.wt) | 2.25 ± 0.010**a | 1.48 ± 0.01**a | 1.37 ± 0.02**a | 1.29 ± 0.024*a | 40.55 |
| Indomethacin (10mg/kg bd. wt) | 2.34 ± 0.022** | 1.50 ± 0.01** | 1.44 ± 0.01** | 1.07 ± 0.013** | 50.69 |
Table1: Effect of NE-AP on carageenan-induced rat paws odema.
| Change in paw valume (mL) at | Percentage inhibition at 4 h | ||||
| Groups/ Treatment | 1 h | 2 h | 3 h | 4 h | |
| Disease control | 2.77 ± 0.03 | 2.75 ± 0.014 | 2.72 ± 0.010 | 2.70 ± 0.04 | - |
| NE-AP (50mg/kg bd.wt) | 2.63 ± 0.009**a | 2.41 ± 0.01**a | 2.21 ± 0.010**a | 1.73 ± 0.013**a | 35.95 |
| NE-AP (100 mg/kg bd. wt) | 2.65 ± 0.010**a | 1.48 ± 0.01**a | 1.37 ± 0.02**a | 1.39 ± 0.024*a | 48.51 |
| Indomethacin (10mg/kg bd. wt) | 2.71 ± 0.022** | 2.30 ± 0.01** | 2.05 ± 0.016** | 1.19 ± 0.01** | 55.92 |
Table 2: Effect of NE-AP on formalin-induced paw odema.
Dose 100 mg/kg bd. wt of NE-AP was found to be more potent as compared with 50 mg/kg bd. wt. In cotton pellet granuloma, dry weight of cotton pellet granuloma was 24.21 mg, 19.02 mg and 16.02 mg in NE-AP (50 mg/kg bd. wt.), NE-AP (100 mg/kg bd. wt.) and indomethacin treated group respectively. Significant (P<0.01) decrease in granuloma was observed in NE-AP (50 mg/kg bd. wt.), NE-AP (100 mg/kg bd. wt.) and indomethacin treated group (Table 3).
| Group/Treatment | Dry weight (mg) granuloma | Percentage inhibition of granuloma formation |
|---|---|---|
| Normal control | 37.01 ± 0.426 | - |
| NE-AP (50 mg/kg bd. wt.) | 24.21 ± 0.40**a | 34.58 |
| NE-AP (100 mg/kg bd. wt.) | 19.02 ± 0.833**a | 48.59 |
| Indomethacin (10 mg/kg) | 16.024 ± 0.217** | 56.7 |
Table 3: Effect of NE-AP on cotton pellet -induced granuloma in rats.
NE-AP showed significant anti-inflammatory activity with carragenan induced, formalin induced, and cotton pellet granuloma, hence can be used in treatment of acute and subacute inflammation. As preliminary Phytochemical analysis showed the presence of flavonoids, triterpenoids, steroids and tannins. Triterpenoids, Flavonoids and steroids are reported to release elastase by human neutrophils which indicate anti-inflammatory activity [43- 45]. Anti-inflammatory activity of NE-AP might be due to inhibition of chemical mediators viz. prosthaglandin.
Conclusion
In conclusion, the present study indicates Nano formulation of Abrus precatorius root extract (NE-AP) showed anti-inflammatory action similar to indomethacin, So NE-AP can be used in the management of acute and subacute inflammation.
Acknowledgement
Authors are thankful to Management and Principal, Gokaraju Rangaraju College of Pharmacy for providing the working facilities for performing the research.
References
- Liao JC, Deng JS, Chiu CS, et al. Chemical compositions, anti-inflammatory, antiproliferative and radical-scavenging activities of Actinidia callosa var. ephippioides. Am J Chinese Med 2012; 40:1047-1062.
- Sen S, Chakraborty R, Sridhar C, et al. Free radicals, antioxidants, diseases and phytomedicines: current status and future prospect. Int J Pharm Sci Review Res 2010; 3:91-100.
- Vonkeman HE, Brouwers JR, van de Laar MA. Understanding the NSAID related risk of vascular events. BMJ 2006; 332:895-898.
- Sajeesh T, Parimelazhagan T. Analgesic, anti-inflammatory, and GC-MS studies on Castanospermum australe A. Cunn. & C. Fraser ex Hook. Scientific World J 2014; 1:2014.
- Aparna Mani KM, Seethalakshmi S, Gopal V. Evaluation of In-vitro Anti-Inflammatory Activity of Silver Nanoparticles Synthesised using Piper Nigrum Extract. J Nanomed Nanotech 2015; 6:268–273.
- Da Silva JB, Temponi Vdos S, Gasparetto CM, et al. Vernonia condensata Baker (Asteraceae): a promising source of antioxidants. Oxid Med Cell Longev 2013; 698018.
- Bonifácio BV, da Silva PB, dos Santos Ramos MA, et al. Nanotechnology-based drug delivery systems and herbal medicines: A review. Int J Nanomed 2014; 9:1.
- Jiang W, Kim BY, Rutka JT, et al. Advances and challenges of nanotechnology-based drug delivery systems. Exp Opinion Drug Delivery 2007; 4:621-33.
- Servat-Medina L, González-Gómez A, Reyes-Ortega F, et al. Chitosan–tripolyphosphate nanoparticles as Arrabidaea chica standardized extract carrier: Synthesis, characterization, biocompatibility, and antiulcerogenic activity. Int J Nanomed 2015; 10:3897.
- Gopinath SM, Saha NS, John VJ, et al. Biological Synthesis Characterization and Application of Silver Nano Particles A Review. Int J Pharm App 2013; 4:19–28.
- Kedi PB, Meva FE, Kotsedi L, et al. Eco-friendly synthesis, characterization, in vitro and in vivo anti-inflammatory activity of silver nanoparticle-mediated Selaginella myosurus aqueous extract. Int J Nanomed 2018; 13:8537.
- Ghaffari-Moghaddam M, Hadi-Dabanlou R, Khajeh M, et al. Green synthesis of silver nanoparticles using plant extracts. Korean J Chem Eng 2014; 31:548-557.
- Galdiero S, Falanga A, Vitiello M, et al. Silver nanoparticles as potential antiviral agents. Molecules 2011; 16:8894-918.
- Meva FE, Ebongue CO, Fannang SV, et al. Natural substances for the synthesis of silver nanoparticles against Escherichia coli: The case of Megaphrynium macrostachyum (Marantaceae), Corchorus olitorus (Tiliaceae), Ricinodendron heudelotii (Euphorbiaceae), Gnetum bucholzianum (Gnetaceae), and Ipomoea batatas (Convolvulaceae). J Nanomaterials 2017; 1:2017.
- Teja K, Satyanarayana T, Saraswathi B, et al. Phytochemical and In vitro anti-inflammatory activity on abrus precatorius. Asian J Res Pharm Sci 2019; 9:50-54.
- Dhawan BN, Patnaik GK, Rastogi RP, et al. Screening of Indian plants for biological activity. Indian J Exp Biol 1977.
- Arora R. Gill NS, Kaur S, et al. Phytopharmacological Evaluation of Ethanolic Extract of the Seeds of Abrus precatorius Linn. J Pharmacol Toxicol 2011; 6:580-588.
- Premanand R, Ganesh T. Neuroprotective effects of Abrus precatorius Linn. aerial extract on hypoxic neurotoxicity induced rats. Int J Chem Pharmac Sci 2010; 1:9-15.
- Gogte VM. Ayurvedic pharmacology and therapeutic uses of medicinal plants (Dravyagunavigyan), Bharatiya Vidya Bhavan (SPARC).
- Sethi N, Nath D, Singh RK. Teratological aspects of Abrus precatorius seeds in rats. Fitoterapia 1990; 61:61-63.
- Agarwal SS, Ghatak N, Arora RB, et al. Antifertility activity of the roots of Abrus precatorius, Linn. Pharmacol Res Communications 1970; 2:159-163.
- Zia-Ul-Haque A, QaziM H, Hamdard ME. Studies on the antifertility properties of active components isolated from the seeds of Abrus precatorius Linn. Pakistan J Zool 1983; 15:129139.
- Georgewill OA, Georgewill UO. Evaluation of the anti-inflammatory activity of extract of Abrus precatorious. Eastern J Med 2009; 14:23.
- Khaleqe A, Aminuddin M, Mulk SAU. Investigations of Abrus precatorius L. constituents of dry root. Pak C S I R Bull Monogra 1966; 3:203.
- Willaman JJ, Schubert B. Alkaloid-bearing plants and their contained alkaloids. US Department of Agriculture 1961.
- Ibrahim N. Phytochemical studies of Abrus precatorius alkaloids. Herba Hung 1980; 19:21-26.
- Ghosal S, Dutta SK. Alkaloids of Abrus precatorius. Phytochemistry 1971; 10:195-198.
- Akinloye BA, LA A. Abrus precatorius leaves-a source of glycyrrhizin. Niger J Pharm 1981; 12:405.
- Kuo SC, Chen SC, Chen LH, et al. Potent antiplatelet, anti-inflammatory and antiallergic isoflavanquinones from the roots of Abrus precatorius. Planta Med 1995; 61:307-312.
- Chiang TC, Chang HM, MAK TW. New oleanene type triterpenes from Abrus precatorius and X-ray crystal structure of abrusgenic acid-methanol 1: 1 solvate. Planta Med 1983; 49:165-169.
- Chang HM, Chiang TC, Mak TC. Isolation and structure elucidation of abruslactone a: A new oleanene-type triterpene from the roots and vines of Abrus precatorius L. J Chem Society Chem Commun 1982; 1197-1198.
- Choi YH, Hussain RA, Pezzuto JM, et al. Abrusosides AD, four novel sweet-tasting triterpene glycosides from the leaves of Abrus precatorius. J Natural Products 1989; 52:1118-1127.
- Choi YH, Kinghorn AD, Shi X, et al. Abrusoside A: A new type of highly sweet triterpene glycoside. J Chem Society Chem Commun 1989; 887-888.
- Saxena VK, Sharma DN. A new isoflavone from the roots of Abrus precatorius. Fitoterapia 1999; 70:328-329.
- Hongfang, Yang Hui, Wang Chuang. Controllable preparation and mechanism of nano-silver mediated by the microemulsion system of the clove oil. Results Phys 2017; 7:3130-3136.
- Qu J, Zhang L, Chen Z, et al. Nanostructured lipid carriers, solid lipid nanoparticles, and polymeric nanoparticles: which kind of drug delivery system is better for glioblastoma chemotherapy? Drug Deliv 2016; 23:3408-3416.
- Kiran Yadav, Deepak Yadav, Manisha Yadav, et al. Noscapine loaded PLGA Nanoparticles prepared using oil-in-water emulsion solvent evaporation method. J Nanopharmaceu Drug Deliv 2015; 3:1-9.
- Ramesh G, Sathesh Kumar S. Formulation and characterization of Noscapine-Loaded poly-caprolactone nanoparticles. Asian J Pharmaceu 2019; 13,10-17.
- Winter CA, Risley EA, Nuss GW. Carrageenin-induced edema in hind paw of the rat as an assay for antiinflammatory drugs. Proceedings of the society for experimental biology and medicine 1962; 111:544-547.
- Winter CA, Porter CC. Effect of alterations in side chain upon anti-inflammatory and liver glycogen activities of hydrocortisone esters. J Am Pharm Assoc 1957; 46:515-519.
- Pouton CW. Formulation of poorly water-soluble drugs for oral administration: Physicochemical and physiological issues and the lipid formulation classification system. Eur J Pharm Sci 2006; 29:278-287.
- Conforti A, Franco L, Milanino R, et al. Copper and ceruloplasmin (Cp) concentrations during the acute inflammatory process in the rat. Agents Actions 1982; 12:303-307.
- Kim HP, Son KH, Chang HW, et al. Anti-inflammatory plant flavonoids and cellular action mechanisms. J Pharmacol Sci 2004; 0411110005-.
- Yen CT, Hsieh PW, Hwang TL, et al. Flavonol glycosides from Muehlenbeckia platyclada and their anti-inflammatory activity. Chem Pharm Bulletin 2009; 57:280-282.
- Nam NH. Naturally occurring NF-κB inhibitors. Mini Reviews Med Chem 2006; 6:945-651.
Author Info
Sneha Nawale*, Sindhu Bhargavi M, Prasanna Laxmi K and Ganga Raju M
Department of Pharmacology, Gokaraju Rangaraju College of Pharmacy, Hyderabad, IndiaCitation: Sneha Nawale, Sindhu Bhargavi M, Prasanna Laxmi K, Ganga Raju M, Development, Characterization and Evaluation of Nanoformulation of Abrus Precatorius Root Extract for Anti Inflammatory Activity, J Res Med Dent Sci, 2021, 9 (4):149-156.
Received: 15-Feb-2021 Accepted: 31-Mar-2021
