Research - (2022) Volume 10, Issue 5
Corrosion Resistance of Titanium-Molybdenum Alloy as Alternative Metals for Endodontic Rotary Instruments (An In Vitro Study)
Mohammad K Sabah* and Mohammed R Hammed
*Correspondence: Mohammad K Sabah, Conservative and Esthetic Dentistry Department, College of Dentistry, University of Baghdad, Iraq, Email:
Abstract
Purpose: Sodium hypochlorite was considered one of the robust irrigant solutions in endodontic treatments. This study evaluates the effect of NaOCl on the beta-titanium and nickel-titanium rotary files. Materials: Two conventional NiTi files (EndoSequence, Endostep), two heat-treated NiTi files (2 Shape, Mono 2 gold), and two Beta-titanium files (TMA alloy) were immersion in NaOCl for 1hr and 24 hr. the corrosion analysis was performed via visual inspection and filed immersion scanning electron microscopy (FE-SEM). Results: Both conventional and heat-treated files were susceptible to NaOCl corrosion. More attacks occurred through visual inspection and FE-SEM analysis with increasing immersion time. The beta-titanium files showed corrosion resistance even with increasing NaOCl immersion time. Conclusion: Within the limitations of this study, beta-titanium files exhibited more corrosion resistance to NaOCl (5.25%) for 1hr and 24hr. NiTi files show less corrosion resistance significantly when increasing the immersion time.
Keywords
Corrosion resistance, Ti-Mo alloys, NiTi alloys
Introduction
The introduction of NiTi rotary instruments has facilitated endodontic procedures by reducing the time for chemo-mechanical preparation minimizing procedural errors associated with hand instrumentation [1]. The metallurgy of endodontic files has grown over the decades. As a result, endodontic files have changed from traditional stainless steel to nickel-titanium (NiTi) alloys, which exhibit more excellent physical properties, including flexibility, high torsional strength, and shape memory capabilities [2,3]. Despite that, the sudden fracture of the nickel-titanium (NiTi) alloys still occurs clinically. Therefore, it is essential to investigate different alloys with different properties for manufactured endodontic instruments [4].
In orthodontics, the archwires is fabricated from different alloys such as titanium-molybdenum (Ti-Mo) alloy, known as β-Titanium or TMA©. This alloy was introduced to orthodontics in 1980 and used during orthodontics treatment. It has been used because it has an intermediate modulus of elasticity between Ni-Ti and stainless steel. Furthermore, it may be helpful in Nickel allergic patients [4-6]. The root canal system complexity cannot be clean totally by mechanical canal preparation. Successful root canal treatment depends on mechanical and chemical debridement. The most common irrigating solution is NaOCl, at different concentrations (0.5%- 5.5%). It has a unique tissue-dissolving property and a broad antimicrobial spectrum [7-11].
The endodontic files repetitively interact with NaOCl inside the root canal space during the root canal preparation. Multiple cycles of cleaning and disinfection could be affected the endodontic file. NaOCl may corrode stainless steel and NiTi files at different ratios of 0.5- 5.5% as it is a highly alkaline solution [12,13]. This study aimed to analyze in vitro the corrosion susceptibility of (Ni-Ti) and (Ti-Mo) alloys as an endodontic instrument immersed in 5.25% NaOCl by visual assessment and Filed Immersion scanning electron microscopy (FE-SEM).
Materials and Method
Samples distribution
Sixty new endodontic files were used in this study divided into two groups symmetrical and asymmetrical. Symmetrical groups are included subgroup A (EndoSequence, Bressler, USA), subgroup B (Endostep, Perfect, China), subgroup C (TMA, IOS, USA). In contrast, asymmetrical groups are included subgroup D (2shape, Micromega, France), subgroup E (Mono 2 gold, Perfect, China), and subgroup F (TMA, IOS, USA). Ten files for each subgroup were divided according to immersion time in sodium hypochlorite solution. Five files were immersed in 5.25% NaOCl for (1 hr) and the other five files for (24 hr) (Figure 1).
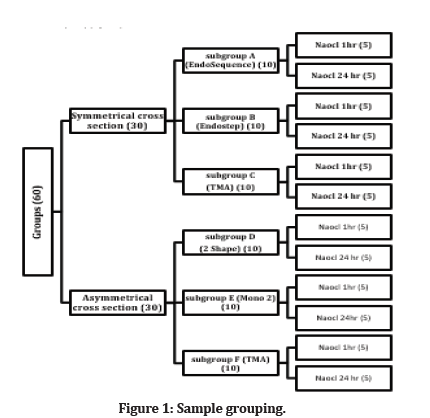
Figure 1: Sample grouping.
Methodology
The Eppendorf was open and filled with (0.75 mL) of 5.25% NaOCl (CHLORAXID, CERKAMED), and the flute and shaft were immersed approximately 18 mm of the file. To simulate the clinical situation, all samples were placed in an incubator at 37 °C (Figure 2).
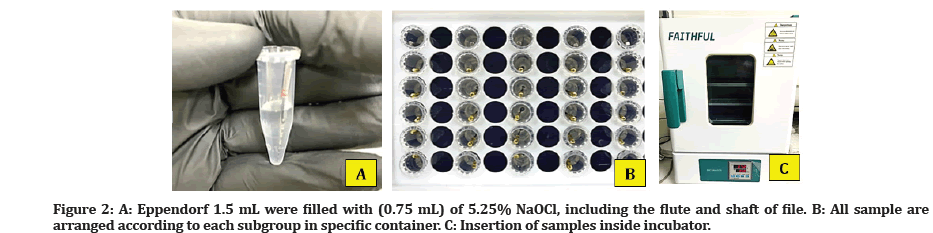
Figure 2: A: Eppendorf 1.5 mL were filled with (0.75 mL) of 5.25% NaOCl, including the flute and shaft of file. B: All sample are arranged according to each subgroup in specific container. C: Insertion of samples inside incubator.
The samples put in front of a white background were used to visualize all the samples via the naked eye. The Eppendorf were monitored after (1 hr) and (24 hr), and the samples were evaluated for color changes, the presence of bubbles, and precipitate formation in the solutions. Each file was taken out of the Eppendorf after incubation, then washed in distilled water double, and allowed to air-dry at room temperature overnight. Then randomly selected three files from each subgroup and mounted them on an FE-SEM platform, and the surfaces were examined using FE-SEM at different magnifications (Figure 3).
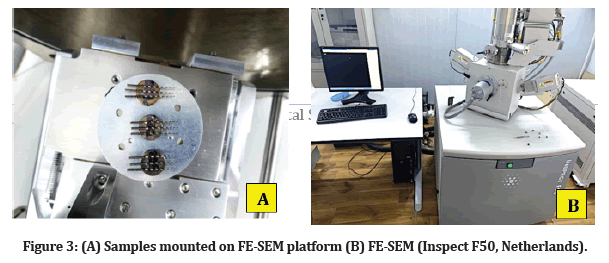
Figure 3: (A) Samples mounted on FE-SEM platform (B) FE-SEM (Inspect F50, Netherlands).
Results
Visual Inspection of the Samples in Sodium Hypochlorite
The following parameters (color, bubble, and volume) were observed during the corrosion process. Color of NaOCl changed by starting black particles precipitation after (1 hr) in subgroups A and subgroup E; then the color becomes more intense after 24 hr for subgroups A, D, and E. Furthermore, the bubbles inside the NaOCl started after 1hr of immersion then increased over time to become more significant after 24 hr in the following subgroups A, D, and E with decreasing of the volume of NaOCl over the time as a result of corrosion reaction (Figure 4).
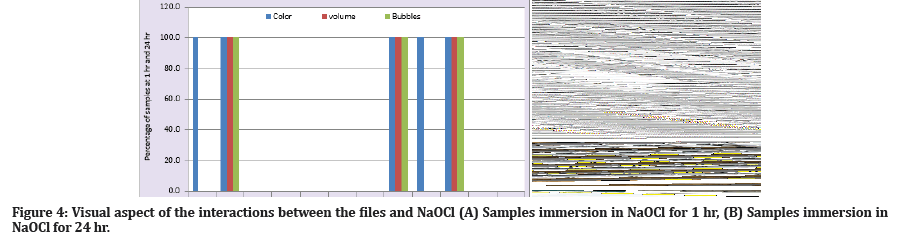
Figure 4: Visual aspect of the interactions between the files and NaOCl (A) Samples immersion in NaOCl for 1 hr, (B) Samples immersion in NaOCl for 24 hr.
At (1 hr) immersion inside the NaOCl, the percentage of precipitation formation was for subgroups A and E were (100%) and for subgroups B, C, D, and F were (0%) with no bubble formation and no volume changes for all subgroups. While at the (24 hr), the precipitation formation, bubbles formations, and volume changes were for subgroups A, D, E were (100%), while for subgroups B, C, and F were (0%) (Figure 5).
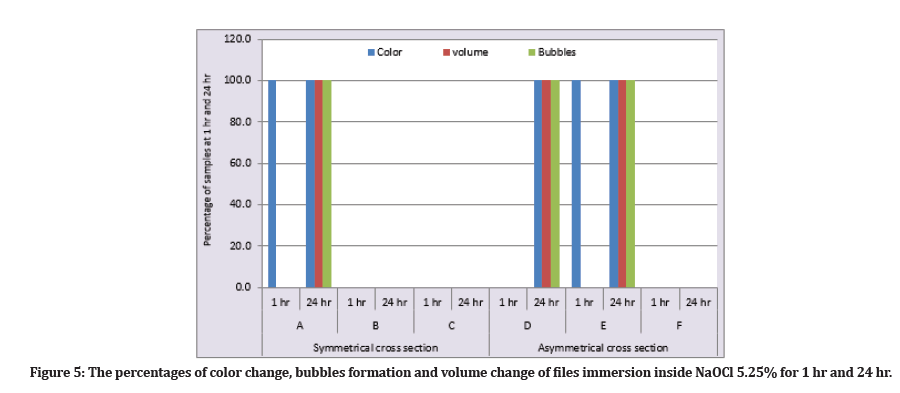
Figure 5: The percentages of color change, bubbles formation and volume change of files immersion inside NaOCl 5.25% for 1 hr and 24 hr.
FE-SEM analysis
The surface micro-morphology of the files was screened via FE-SEM for randomly selected three samples from each subgroup. Each piece was examined from the shank to the tip at different magnifications (Figure 6).
Figure 6: Three samples from each subgroup over the FE-SEM platform to scanning from the shank to the tip at different magnifications.
1-Control subgroup (non-exposed files)
The three unused, brand-new files from each subgroup were screened under FE-SEM inspection showed that no single file system was absolutely free of defects or debris (Figure 7).
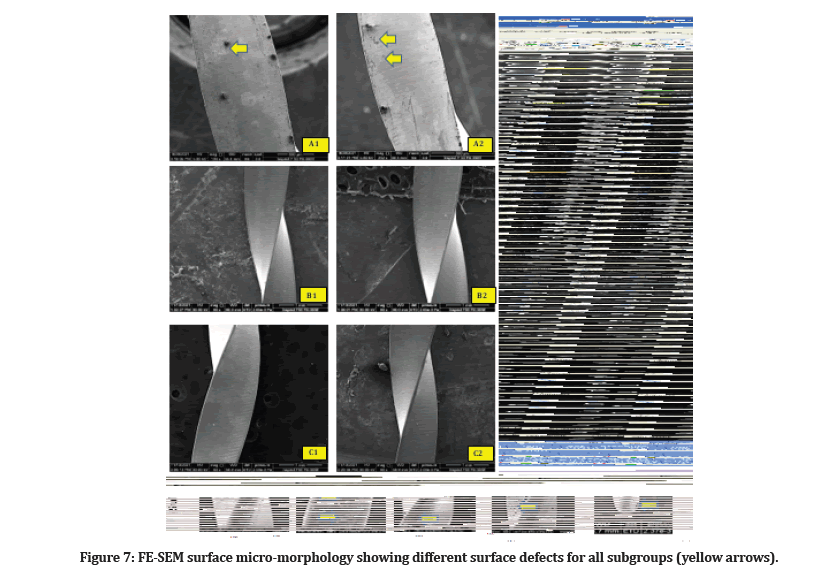
Figure 7:FE-SEM surface micro-morphology showing different surface defects for all subgroups (yellow arrows).
2- Immersion for (1 hr) in NaOCl
Compared to the control subgroup, there are signs of corrosion identified under FE-SEM for the (subgroups A and E) that appear as white precipitation and black pitting over the surfaces (Figure 8).
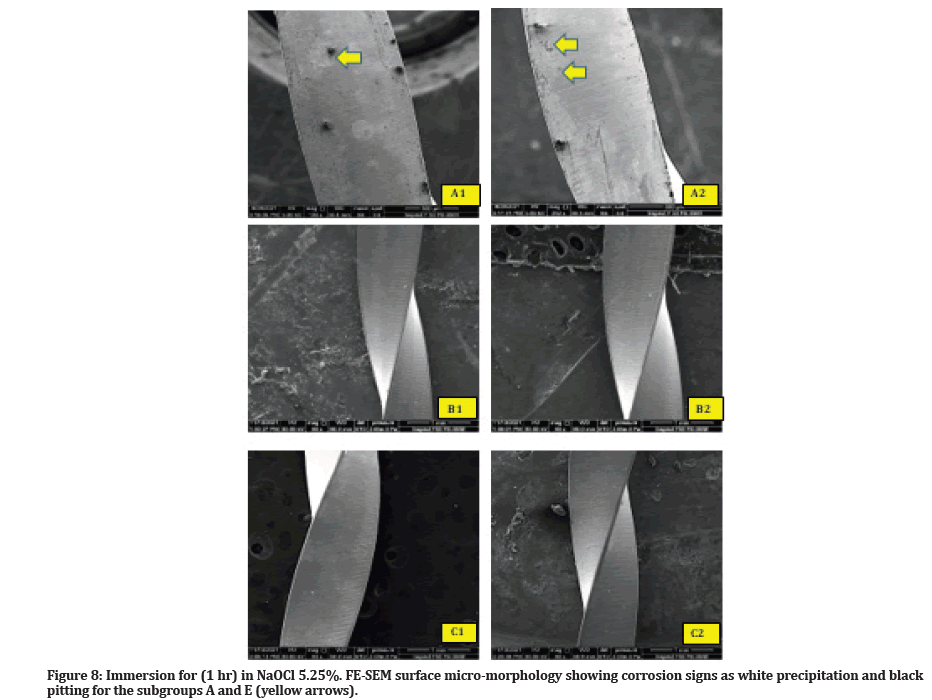
Figure 8:Immersion for (1 hr) in NaOCl 5.25%. FE-SEM surface micro-morphology showing corrosion signs as white precipitation and black pitting for the subgroups A and E (yellow arrows).
3- Immersion for (24 hr) in NaOCl
The more aggressive action of NaOCl was shown on subgroups A, D, and E. FE-SEM shows a lot of white precipitations, corrosive pitting, and metal surface damaging (Figure 9).
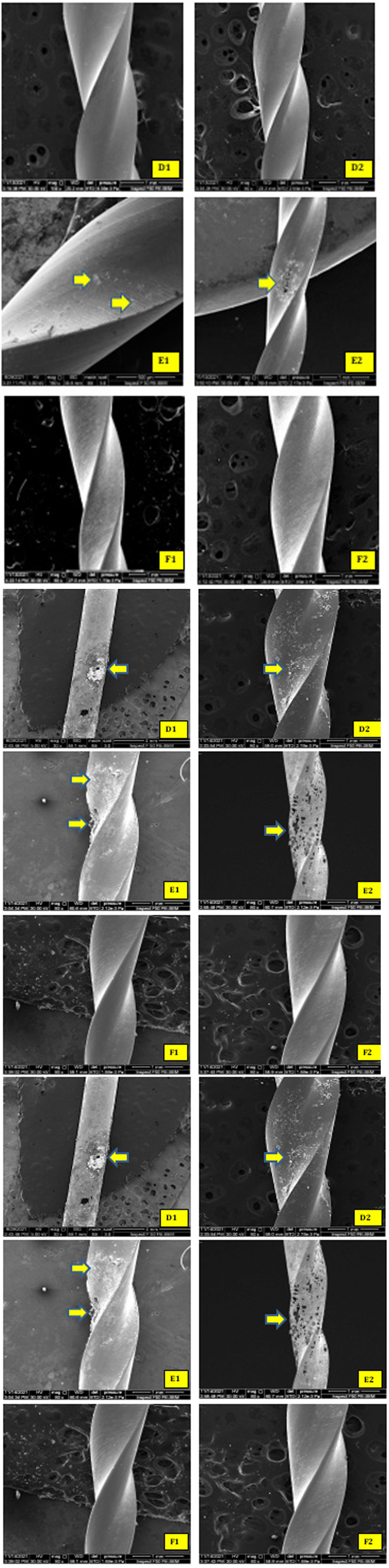
Figure 9:Immersion for (24 hr) in NaOCl 5.25%. FE-SEM surface micro-morphology showing white precipitation, corrosion pitting for the subgroups A and D and sever metal surface damaging in subgroup E (yellow arrows).
Discussion
Titanium molybdenum alloy, known as β-Titanium or TMA©, is used in orthodontic therapy. Most corrosion studies on Ti-Mo alloy were examined in acidic artificial saliva, mouth rinsing with fluoride-containing products [14-16]. This study compared the effect of NaOCl (5.25%) on TMA alloy as an endodontic rotary file with other conventional and heat-treated Ni-Ti rotary files by using direct visual inspection and FE-SEM analysis. The more extended NaOCl immersion of samples is associated with increasing the black precipitate inspection, which agrees with the study of O’Hoy et al., 2003 [17]. The levels of corrosion resistance of the endodontic file systems can be arranged from most resistant to least as follows: subgroup C, subgroup F, subgroup B, subgroup A, subgroup D, and subgroup E, according to our observation.
The present study found that TMA endodontic rotary files in both cross-sections (symmetrical and asymmetrical) (subgroups C and F) showed excellent corrosion resistance when immersion in NaOCl for (1hr and 24hr). The possible explanation is that TMA alloy is free from Nickel elements. The selective removal of the nickel from the instrument’s surface by NaOCl immersion may cause the micro-pitting that has an adverse effect on the NiTi files [18]. Another possible explanation is that TMA alloy is rich in Molybdenum elements. Molybdenum is a silvery-white metal that is ductile and highly resistant to corrosion [19].
Regarding NiTi rotary files, the presented study shows that in symmetrical cross-sections. Subgroup A has less corrosion resistance when increasing the immersion time in NaOCl for (24 hr) even this subgroup has electropolishing surface treatment. This may be attributed to these files being considered a conventional NiTi file. So, these instruments have to be ground rather than twisted. So, the surface irregularities such as milling mark potentially boost corrosion susceptibility [20]. This result agrees with Cheung et al., 2007; they are shown that the corrosion resistance doesn’t improve by electropolishing of strain-cycled instruments [21]. However, this result disagrees with Praisarnti et al., 2010 [22].
Conversely, subgroup B showed more corrosion resistance than (subgroup A) in both (1 hr and 24 hr) immersion in NaOCl. This may be related to the passivation layer capacity in supporting and protecting the external surface from aggressive pitting corrosion [23].
On the other hand, in asymmetrical subgroups, subgroup E is minor corrosion resistant than subgroup D, even though they have the surface heat treatment. The possible explanations are that pitting corrosion may be more closely related to the endodontic file's design than the use of heat treatment. They are variable in the design along the axis of the instrument. The other cause may be related to principles of thermodynamics (enthalpy and entropy). ‘’A thermodynamic quantity representing the unavailability of a system's thermal energy for conversion into mechanical work, often interpreted as the degree of disorder or randomness in the system’’. So, molecular disorganization means significant irregularities, directly influencing the corrosion resistance of the material [24,25].
On another side, the effects of NaOCl immersion were evaluated via FE-SEM analysis. It is essential to mention that none of the samples are utterly free of imperfections or debris under FE-SEM examination (Figure 7).
Nevertheless, it can only examine one side of the file at a time, and this is one of FE-SEM limitations. This could explain why minor signs of corrosion were identified on the file surface for the subgroup's A and E that immersion in NaOCl for (1 hr) (Figure 8 - A1, A2; E1, E2). Furthermore, this FE-SEM identification was genuinely reflected in visual inspection of the samples through the formation of fewer black particulates in the bottom of Eppendorf (Figure 4-A).
In the subgroups that immersion in NaOCl for (24 hr), FE-SEM analysis was showing more corrosion aggressive marks on file surfaces, especially for heat-treated subgroups (subgroup D and E) (Figure 9 - D1, D2; E1, E2). The surface is damaging, reflected through the increase of black particulates in the bottom of Eppendorf (Figure 4-B). These findings agree with the study of Lin et al., 2021; they conclude that heat treatment of the NiTi file may not directly impact the file’s susceptibility to NaOCl corrosion [26].
Conclusion
Within the limitation of this study, TMA files in symmetrical and asymmetrical cross-sections have high corrosion resistance when immersion in NaOCl for (1 hr and 24 hr). Ni-Ti files show less corrosion resistance with increased NaOCl immersion time. Heat surface treatment doesn’t prevent the corrosions attack.
References
- Peters OA. Current challenges and concepts in the preparation of root canal systems: A review. J Endod 2004; 30:559-67.
- Glosson CR, Haller RH, Dove SB, et al. A comparison of root canal preparations using Ni-Ti hand, Ni-Ti engine-driven, and K-Flex endodontic instruments. J Endod 1995; 21:146-51.
- Walia H, Brantley WA, Gerstein H. An initial investigation of the bending and torsional properties of Nitinol root canal files. J Endod 1988; 14:346-51.
- Nino-Barrera JL, Aldana-Ojeda L, Gamboa-Martinez LF, et al. Comparison of mechanical and structural properties of nickel-titanium alloy with titanium-molybdenum alloy and titanium-niobium alloy as potential metals for endodontic files. Iranian Endod J 2021; 16:49-55
- Burstone CJ, Goldberg AJ. Beta titanium: a new orthodontic alloy. Am J Orthod 1980; 77:121-32.
- Nordstrom B, Shoji T, Anderson WC, et al. Comparison of changes in irregularity and transverse width with nickel-titanium and niobium-titanium-tantalum-zirconium archwires during initial orthodontic alignment in adolescents: A double-blind randomized clinical trial. Angle Orthod 2018; 88:348-54.
- Zehnder M. Root canal irrigants. J Endod 2006; 32:389-98.
- Hand RE, Smith ML, Harrison JW. Analysis of the effect of dilution on the necrotic tissue dissolution property of sodium hypochlorite. J Endod 1978; 4:60-4.
- Best M, Springthorpe VS, Sattar SA. Feasibility of a combined carrier test for disinfectants: Studies with a mixture of five types of microorganisms. Am J Infect Control. 1994; 22:152-62.
- Rutala WA. APIC guideline for selection and use of disinfectants. Am J Infect Control 1996; 24:313-42.
- Pashley EL, Birdsong NL, Bowman K, et al. Cytotoxic effects of NaOCl on vital tissue. J Endod 1985; 11:525-8.
- Stokes OW, Di Fiore PM, Barss JT, et al. Corrosion in stainless-steel and nickel-titanium files. J Endod 1999; 25:17-20.
- Edie JW, Andreasen GF, Zaytoun MP. Surface corrosion of nitinol and stainless steel under clinical conditions. Angle Orthod 1981; 51:319-24.
- Castro SM, Ponces MJ, Lopes JD, et al. Orthodontic wires and its corrosionâ??The specific case of stainless steel and beta-titanium. J Dent Sci 2015; 10:1-7.
- Pulikkottil VJ, Chidambaram S, Bejoy PU, et al. Corrosion resistance of stainless steel, nickel-titanium, titanium molybdenum alloy, and ion-implanted titanium molybdenum alloy archwires in acidic fluoride-containing artificial saliva: An in vitro study. J Pharm Bioallied Sci 2016; 8:S96.
- Pataijindachote J, Juntavee N, Viwattanatipa N. Corrosion analysis of orthodontic wires: An interaction study of wire type, pH and immersion time. Adv Dent Oral Health. 2018; 10:1-7.
- O'hoy PY, Messer HH, Palamara JE. The effect of cleaning procedures on fracture properties and corrosion of NiTi files. Int Endod J 2003; 36:724-32.
- Pedullà E, Benites A, La Rosa GM, et al. Cyclic fatigue resistance of heat-treated nickel-titanium instruments after immersion in sodium hypochlorite and/or sterilization. J Endod 2018; 44:648-53.
- Considine GD. Molybdenum. Van Nostrand's encyclopedia of chemistry. 5th edn: Wiley-Interscience; 2005: 1038-40.
- Thompson SA. An overview of nickelâ??titanium alloys used in dentistry. Int Endod J 2000; 33:297-310.
- Cheung GS, Shen Y, Darvell BW. Does electropolishing improve the low-cycle fatigue behavior of a nickelâ??titanium rotary instrument in hypochlorite?. J Endod 2007; 33:1217-21.
- Praisarnti C, Chang JW, Cheung GS. Electropolishing enhances the resistance of nickel-titanium rotary files to corrosionâ??fatigue failure in hypochlorite. J Endod 2010; 36:1354-7.
- Bonaccorso A, Tripi TR, Rondelli G, et al. Pitting corrosion resistance of nickelâ??titanium rotary instruments with different surface treatments in seventeen percent ethylenediaminetetraacetic acid and sodium chloride solutions. J Endod 2008; 34:208-11.
- Sonntag RE GJ. Introduction to thermodynamics: classical and statistical. In: 3rd, editor.: John Wiley & Sons, Inc; 1991: 800.
- Lambert FL. Configurational entropy revisited. J Chem Educ 2007;84:1548.
- Lin JH, Karabucak B, Lee SM. Effect of sodium hypochlorite on conventional and heat-treated nickel-titanium endodontic rotary instrumentsâ??An in vitro study. J Dent Sci 2021; 16:738-43.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Mohammad K Sabah* and Mohammed R Hammed
1Conservative and Esthetic Dentistry Department, College of Dentistry, University of Baghdad, IraqCitation: Mohammad K Sabah, Mohammed R Hammed, Corrosion Resistance of Titanium-Molybdenum Alloy as Alternative Metals for Endodontic Rotary Instruments (An In Vitro Study), J Res Med Dent Sci, 2022, 10 (5): 124-131.
Received: 02-May-2022, Manuscript No. JRMDS-22-62395; , Pre QC No. JRMDS-22-62395 (PQ); Editor assigned: 04-May-2022, Pre QC No. JRMDS-22-62395 (PQ); Reviewed: 18-May-2022, QC No. JRMDS-22-62395; Revised: 23-May-2022, Manuscript No. RMDS-22-62395 (R); Published: 30-May-2022
