Research - (2023) Volume 11, Issue 7
Correlation of Irisin with the Inflammatory Parameters and Liver Function in Rheumatoid Arthritis Patients.
Zainab Qasim Muhammad Al-Yasiri* and Khalid Gatee Al-Fartosi
*Correspondence: Zainab Qasim Muhammad Al-Yasiri, Department of Biology, College of Science, University of Thi Qar, Nasiriyah, Iraq, Email:
Abstract
This study was designed with the aim of investigating the relationship of irisin with some biochemical and inflammatory parameters, among patients with rheumatoid arthritis in Thi Qar Governorate/Iraq. The study included 100 women with rheumatoid arthritis diagnosed by a specialist doctor, their ages ranged between 30-80 years. With 50 healthy women of the same age representing a control group, Blood samples were used for examine the Erythrocyte Sedimentation Rate (ESR), and serum which used to measure Rheumatoid Factor (RF), Anti-Cyclic Citrullinated Peptides (ACCP), irisin, Also, a liver function test, as well as inflammatory Parameters. The results of our study showed a significant decrease (p≤0.05) in the level of irisin in patients compared to the control group. On other hand, the second age group (GII≥50 years) of patients showed a significant decrease (p≤ 0.05) in the concentration of irisin compared to the first age group (GI<50 years). Also the results recorded a significant increase (p≤ 0.05) in the levels of RF and ACCP in the patients compared to the control group. While, we note that there is no significant difference (P>0.05) in the concentration of RF and ACCP for patients when comparing the age groups. In contrast, If we look at the liver function test, we notice that there is a significant increase (p≤0.05) in the concentration of ALP and GPT, while there was no significant difference (P>0.05) in the concentration of GOT in the patients compared to the control group. While, based on the age group, the results showed that, ALP concentration was significantly increased (p≤0.05) in the second group of patients (GII≥50 years) compared to the first group (GI<50 years). In contrast, no significant differences (P>0.05) are observed in the GPT and GOT concentration when comparing the two groups of patients with each other. On the other hand, when we talk about the inflammatory parameters under study, we find that, IL-6, CRP and ESR, were significantly increased (p≤0.05) in patients compared to the control group. In addition to, when comparing patients, based on age groups, we find that ESR value, has significantly increased (p≤0.05) in the second age group of patients (GII≥50 years) compared to the first group (GI<50 years), while, the rest of the inflammatory parameters did not show any Significant differences (P>0.05). Finally, Correlation analysis of the results obtained from the current study on patients with rheumatoid arthritis indicated a negative correlation between irisin with RF, ACCP, GPT, GOT, ALP, IL-6, CRP and ESR..
Keywords
Irisin, Inflammatory parameters, Erythrocyte Sedimentation Rate
Introduction
Rheumatoid Arthritis (RA) is a chronic systemic inflammatory autoimmune disease, generally affecting the peripheral joints of symmetric distribution. It’s characterized by continued chronic inflammation of the synovial joints associated with proliferation of synovial cells and infiltration of active immune inflammatory cells, leading to the progressive destruction of cartilage and bone [1]. Synovitis eventually leads to joint deformities; many of organ systems may be affected. If left untreated therefore, early diagnosis and Treatment of RA can restrict or slow down the joint damage in up to 90% of patients.
It is necessary to note that, rheumatoid arthritis is a lifelong disease the monitored of its progression is according to the criteria of American College of Rheumatology based on changes in specific symptoms and laboratory findings, which include early involvement of many joints, an elevated erythrocyte sedimentation rate, C-reactive protein level, Positive Rheumatoid Factor (RF), and Anti-Citrullinated Protein Antibodies (ACPAs), and early radiological changes [2-4].
It should be noted that, Inflammation in RA is caused by accumulation of autoreactive T cells and B cells in the synovial tissues of patients. T cells are immunologically tolerant to autoantigens; when self-tolerance is broken, autoreactive T cells are activated, and they stimulate B cells to induce production of autoantibodies "Anti-Citrullinated Protein Antibodies (ACPAs) and Rheumatoid Factor autoantibodies (RF)" that form immune complexes with antigens, which are deposited in tissues and activate complements to cause histological damage. These autoantibodies increase the incidence of inflammation either by direct activation of macrophages or by induction of the complement cascade [5].
Irisin is a myokine/adipokine that was recently discovered by Bostrom et al, in Harvard University, Irisin is attracting more and more interest due to the multiple effects it exerts on different tissues such as fat, brain, muscle and bone. Irisin is produced from a transmembrane glycoprotein called fibro Nectin type III Domain-Containing protein 5 (FNDC5), the expression of which is regulated by the peroxisome proliferatoractivated transcriptional receptor gamma-1α (PGC-1α), which is in turn induced by physical activity. It should be noted that, The effect of irisin on adipose tissue, demonstrated by its ability to induce the transformation of white adipose tissue into brown fat cells by enhancing the expression of mitochondrial uncoupling protein 1 (UCP1), Successful evidence has highlighted the widespread effects of irisin in other tissues, such as its role in the regulation of energy metabolism [6,7].
It was found that, Lack of physical activity is a risk factor for many chronic diseases, such as type 2 diabetes, obesity, immune dysfunction, asthma, and coronary heart disease. Most of these diseases are associated with chronic inflammation. Regular and moderate physical activity favorably affects the functioning of the immune system and reduces systemic inflammation. Skeletal muscle modulates the inflammatory response primarily by secreting myokine such as irisin [8-10].
Aim
The current study proposed to highlight the following points
Measuring the level of irisin for RA patients.
Investigate of irisin level relationship with RF and ACCP.
Evaluate the association of irisin level with Liver enzymes.
Assessment the correlation of irisin level with inflammatory parameters [11].
Materials and Methods
Subjects
This study targeted 150 women including 100 women with rheumatoid arthritis, and 50 healthy women as a control group. Their ages ranged between 30-80 years this study conducted in specialized outpatient clinics in Thi Qar Governorate / Iraq, during the period from November 2020 to July 2021.
Rheumatoid arthritis cases were diagnosed by a medical specialist. The other group represents apparently healthy individuals as a control group.
Depending on age, patients were divided equally into two age groups (GI < 50 years and GII ≥ 50 years). The same was true for healthy controls [12].
Blood Collection
Approximately (5 ml) of venous blood sample was withdrawn for both RA patients and the control group, Each sample was divided into two parts: 2 ml placed in a tube containing an anticoagulant (EDTA tube) used to examine erythrocyte sedimentation rate (ESR), The remaining 3 ml was placed in anticoagulant-free tube, to obtain serum, which was separated after centrifugation at 3000 rpm for 10 minutes. the serum was used for biochemical and inflammatory tests [13].
Immunological Parameters
Determining of Rheumatoid Factor (RF)
RF is important in the diagnosis of rheumatoid arthritis, So, The quantitative immunological determination of human rheumatoid factors in serum on COBAS INTEGRA systems. The Roche RF assay is based on the immunological agglutination principle with enhancement of the reaction by latex.
Immunoturbidimetric assay Latex‑bound heat‑inactivated IgG (antigen) reacts with the RF‑antibodies in the sample to form antigen/antibody complexes which, following agglutination, are measured turbid metrically [14-17].
Determination of Anti-Cyclic Citrullinated Peptides (ACCP)
Citrulline has been identified as a target of a whole group of autoantibodies such as Anti-Perinuclear Factor (APF), Anti-Keratinocyte (AKA), and Anti- filaggrin (AFA) antibodies. Its detection in the serum of rheumatoid arthritis patients led to the development of anti-CCP assays that possess a high specificity for RA [18].
In 2010, the American College of Rheumatology (ACR) and the European League Against Rheumatology (EULAR) developed new classification criteria for rheumatoid arthritis to improve early diagnosis. Anti- CCP assay has been added as a diagnostic component for RA, which can be measured by designing an electro Chemilumniscence immunoassay “ECLIA” using a Cobas e 411 immunoassay analyzer.
Biochemical Parameters
Irisin Level Determination
This kit is an Enzyme-Linked Immunosorbent Assay (ELISA). The plate has been pre-coated with human Irisin antibody. Irisin present in the sample is added and binds to antibodies coated on the wells. And then biotinylated human Irisin Antibody is added and binds to Irisin in the sample. Then Streptavidin-HRP is added and binds to the Biotinylated Irisin antibody. After incubation unbound Streptavidin-HRP is washed away during a washing step. Substrate solution is then added and color develops in proportion to the amount of human Irisin. The reaction is terminated by addition of acidic stop solution and absorbance is measured at 450 nm [19].
Determination of Liver function test
Aspartate Aminotransferase (AST or GOT)
Method according to the International Federation of Clinical Chemistry (IFCC), with pyridoxal-5'- phosphate.3,4 AST in the sample catalyzes the transfer of an amino group between L‑aspartate and 2‑oxoglutarate to form oxaloacetate and L‑glutamate. The oxaloacetate then reacts with NADH, in the presence of malate dehydrogenase (MDH), to form NAD+. Pyridoxal phosphate serves as a coenzyme in the amino transfer reaction, which ensures full enzyme activation. The rate of the NADH oxidation is directly proportional to the catalytic AST activity. It is determined by measuring the decrease in absorbance at 340 nm [20].
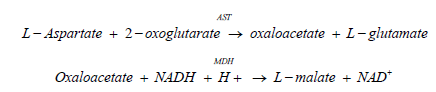
Alanine Aminotransferase (ALT or GPT)
The method according to the International Federation of Clinical Chemistry (IFCC), with pyridoxal-5'-phosphate. ALT catalyzes the reaction between L‑alanine and 2‑oxoglutarate. The pyruvate formed is reduced by NADH in a reaction catalyzed by Lactate Dehydrogenase (LDH) to form L‑lactate and NAD+. Pyridoxal phosphate serves as a coenzyme in the amino transfer reaction that ensures full enzyme activation. The rate of the NADH oxidation is directly proportional to the catalytic ALT activity. It is determined by measuring the decrease in absorbance at 340 nm [21].
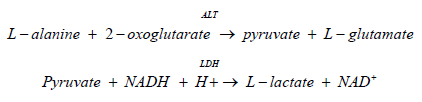
Alkaline Phosphatase (ALP)
Colorimetric assay in accordance with a standardized method In the presence of magnesium and zinc ions, p‑nitro phenyl phosphate is cleaved by phosphatases into p‑nitro phenol and phosphate .The released p‑nitro phenol is directly proportional to the activity of catalytic ALP. It is determined by measuring the increase in absorbance at 409 nm [22].
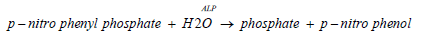
Inflammatory Parameters Determination
Interleukin 6 (IL-6)
This kit is an Enzyme-Linked Immunosorbent Assay (ELISA). The plate has been pre-coated with human IL-6 antibody. when sample adding the IL6 found in it, binds to wells coated antibodies, then adding bio treated human IL-6 Antibody to binds with IL-6 in the sample adding Streptavidin-HRP which binds to the Bio treated IL-6 antibody. After incubation washes away the unbound Streptavidin-HRP during a washing step, the developing colored after adding of substrate solution is proportion to amount of human IL-6 terminating the reaction by adding of acidic stop solution and measuring the absorbance at 450 nm [23-25].
C-Reactive Protein (CRP)
Particle enhanced turbid metric assay, Human CRP agglutinates with latex particles that coated with monoclonal anti‑CRP antibodies. the determination of precipitate can be done turbid metrically at 552 nm, the quantitative immunological determination of C‑reactive protein in human serum on COBAS INTEGRA systems [26].
Erythrocyte Sedimentation Rate (ESR) Evaluation
The Erythrocyte Sedimentation Rate (ESR) expresses in mm per hour the rate at which red blood cells settle when anti-coagulated blood is allowed to stand in a narrow tube (Westergren). It is measured by the height of the column of clear plasma at the end of one hour.
Statistical Analysis
The results were statistically analyzed using the Statistical Package for Social Sciences (SPSS) for windows version 23 using analysis of variance (ANOVA) and t-test means; Standard Deviations (SD) and LSD (Least Significant Differences) were found. To find out the significance of the differences at the significance level (p < 0.05). Correlation analysis was also performed using Pearson's correlation coefficient [27].
Results
Immunological Parameters
Rheumatoid Factor (RF) & Anti-Cyclic Citrullinated Peptides (ACCP)
The results presented in Table 3.1 indicate a significant increase (p≤ 0.05) in both of RF and ACCP concentration in patients compared to their corresponding control groups to each of these parameters [28].
| Groups | Parameters Mean ±SD | |
|---|---|---|
| RF(IU/mL) | ACCP(U/mL) | |
| Control | 5.770b ± 2.239 | 7.310b ± 1.935 |
| Patients | 31.727a ± 13.766 | 125.088a ± 57.860 |
| P- value | 0 | 0 |
The similar letters (a, a): no significant differences, the deferent letters (a, b): a significant differences, (SD): Standard Deviation (P>0.05): no significant difference and (p≤0.05): are significant.
Table 3.1: Level of (RF) and (ACCP) in Rheumatoid arthritis patients and control subjects.
The results recorded a significant increase (p≤ 0.05) in the concentration of RF and ACCP for patients in both age groups (GI<50 years and GII ≥ 50 years) when compared with the control groups, while there were no significant differences (P>0.05) when comparing the age groups of patients among themselves as shown in Table 3.2 [29].
| Age Groups | Parameters Mean ± SD | ||
|---|---|---|---|
| (RF) (IU/mL) | (ACCP) (U/mL) | ||
| (GI) < 50 years | Control | 5.080b ± 2.155 | 6.661b ± 2.274 |
| Patients | 28.768a ± 12.326 | 113.230a ± 80.348 | |
| (GII) ≥ 50 years | Control | 6.461b ± 2.155 | 7.958b ± 1.277 |
| Patients | 34.686a ± 14.627 | 126.945a ± 96.089 | |
| LSD | 7.17 | 83.468 | |
The similar letters (a, a): no significant differences, the deferent letters (a, b): a significant differences, (LSD): Least Significant Difference and (SD): Standard Deviation.
Table 3.2: Level of (RF) and (ACCP) in Rheumatoid Arthritis Patients and Control Subjects According to Age.
Biochemical Parameters
Irisin Level
The results recorded a significant decrease (p≤ 0.05) in the concentration of the irisin hormone in patients with rheumatoid arthritis compared with the control group as shown in Table 3.3 [30].
| Irisin (ng/ml) Mean ± SD | P- value | |
|---|---|---|
| Control | Patients | |
| 20.103a ± 2.126 | 14.489b ± 2.703 | 0 |
The similar letters (a, a): no significant differences, the deferent letters (a, b): a significant differences, (SD): Standard Deviation (P>0.05): no significant difference and (p≤0.05): are significant.
Table 3.3: The level of Irisin in Rheumatoid arthritis patients and control subjects.
| Age Groups | Irisin (ng/ml) Mean ± SD | ||
|---|---|---|---|
| (GI) < 50 years | Control | 21.665a ± 1.737 | |
| Patients | 15.822c ± 2.330 | ||
| (GII) ≥ 50 years | Control | 18.540b ± 1.057 | |
| Patients | 13.155d ± 2.392 | ||
| LSD | 1.352 | ||
The similar letters (a, a): no significant differences, the deferent letters (a, b): a significant differences, (SD): Standard Deviation. (P > 0.05): no significant difference and (p≤ 0.05): are significant.
Table 3.4: The level of Irisin in Rheumatoid arthritis patients and control subjects according to age.
On the other hand, the results showed that age had a negative effect on the concentration of irisin for both patients and the control. The second age group (GII ≥ 50 years) of patients recorded a significant decrease (p≤ 0.05) in the concentration of irisin compared to its counterpart in the first age group (GI < 50 years), this is indicated in Table 3.4 [31].
Liver Function Test
It seems clearly visible that, The results indicated a significant increase (p≤ 0.05) in the concentration of ALP and GPT in the patients group compared to the control group, while there was no significant difference (P> 0.05) in the concentration of GOT between the two groups. As shown in Table 3.5 [32].
| Groups | Parameters Mean ± SD | ||
|---|---|---|---|
| ALP (U/L) | GPT (ALT) (U/L) | GOT (AST) (U/L) | |
| Control | 113.825b | 21.200b | 25.400a |
| ±14.285 | ±3.337 | ± 2.678 | |
| Patients | 148.600a | 31.875a | 28.275a |
| ±28.837 | ±9.174 | ± 6.394 | |
| P- value | 0 | 0 | 0.07 |
The similar letters (a, a): no significant differences, the deferent letters (a, b): a significant differences, (LSD): least significant difference and (SD): Standard Deviation
Table 3.5: Level of Liver enzymes in Rheumatoid arthritis patients and control subjects.
The similar letters (a, a): no significant differences, The deferent letters (a, b): a significant differences, (SD): Standard Deviation. (P > 0.05): no significant difference and (p≤ 0.05): are significant.
On the other hand, depending on the age group, the results of the current study showed that the concentration of ALP increased significantly (p≤ 0.05) in the second group of patients (GII≥50 years) compared with the first group (GI < 50 years). As shown in Table 3.6.
| Parameters Mean ± SD |
Age Groups | LSD | |||
|---|---|---|---|---|---|
| (GI) < 50 years | (GII) ≥ 50 years | ||||
| Control | Patients | Control | Patients | ||
| ALP(U/L) | 104.175c | 129.138b | 123.475b | 168.062a | 11.875 |
| ± 7.066 | ± 15.032 | ± 13.148 | ± 26.106 | ||
| GPT(ALT) (U/L) | 19.650b | 32.375a | 22.750b | 31.375a | 4.979 |
| ± 2.777 | ± 10.522 | ± 3.177 | ± 7.698 | ||
| GOT(AST) (U/L) |
24.800a | 27.814a | 26.600a | 28.137a | 3.496 |
| ± 2.783 | ± 5.594 | ± 1.984 | ± 7.147 | ||
The similar letters (a, a): no significant differences, The deferent letters (a, b): a significant differences, (LSD): Least Significant Difference and (SD): Standard Deviation.
Table 3.6: Level of Liver enzymes in Rheumatoid arthritis patients and control subjects according to age.
In contrast, No significant differences (P>0.05) were observed in the GPT concentration when comparing the two groups of patients with each other. As for the GOT concentration, when we highlight it, we note that no significant difference (P > 0.05) was shown in the two groups under study and in both cases, whether at the level of patients and compared with the control [33].
The similar letters (a, a): no significant differences, the deferent letters (a, b): a significant differences, (LSD): least significant difference and (SD): Standard Deviation.
Inflammatory Parameters
Now, by looking closely at Table 3.7 it seems clear that the inflammatory parameters represented by IL-6, CRP, and ESR, were significantly increased (p≤ 0.05) in patients compared to the control group [34].
| Groups | Parameters Mean ± SD | ||
|---|---|---|---|
| IL-6(ng/L) | CRP(mg/L) | ESR(mm/hr) | |
| Control | 27.050b | 4.312b | 17.268b |
| ±6.342 | ±1.006 | ±3.162 | |
| Patients | 67.459a | 16.174a | 45.518a |
| ±16.759 | ±5.219 | ±12.297 | |
| P- value | 0 | 0 | 0 |
The similar letters (a, a): no significant differences, the deferent letters (a, b): a significant differences, (SD): Standard Deviation. (P > 0.05): no significant difference and (p≤ 0.05): are significant.
Table 3.7: Level of IL-6, CRP, and ESR in Rheumatoid arthritis patients and control subjects.
The similar letters (a, a): no significant differences, the deferent letters (a, b): a significant differences, (SD): Standard Deviation. (P > 0.05): no significant difference and (p≤ 0.05): are significant.
Depending on the age group, if we focus on the ESR value, we find that it has significantly increased (p≤ 0.05) in the second age group of patients (GII ≥ 50 years) compared to the first group (GI < 50 years), while we find that the rest of the inflammatory parameters did not show any significant differences (P>0.05) when comparing the age groups of patients among themselves. This is clearly visible from Table 3.8 [35].
| Parameters Mean ± SD |
Age Groups | LSD | |||
|---|---|---|---|---|---|
| (GI) < 50 years | (GII) ≥ 50 years | ||||
| Control | Patients | Control | Patients | ||
| IL-6 | 24.130b | 66.565a | 29.970b | 68.353a | 9.128 |
| (ng/L) | ± 4.370 | ± 20.299 | ± 6.747 | ± 12.526 | |
| CRP | 3.730b | 15.205a | 4.595b | 17.144a | 2.728 |
| (mg/L) | ± 0.469 | ± 5.873 | ± 0.755 | ± 4.333 | |
| ESR | 15.220c | 40.224b | 19.315c | 50.813a | 5.943 |
| (mm/hr) | ± 1.876 | ± 12.059 | ± 2.859 | ± 10.167 | |
The similar letters (a,a): no significant differences, The deferent letters (a,b): a significant differences, (LSD): least significant difference and (SD): Standard Deviation.
Table 3.8: Level of IL-6, CRP, and ESR in Rheumatoid arthritis patients and control subjects according to age.
The similar letters (a,a): no significant differences, The deferent letters (a,b): a significant differences, (LSD): least significant difference and (SD): Standard Deviation.
Correlation Analysis
Correlation analysis between Irisin and other parameters in Rheumatoid arthritis patients.
Data analysis of the current study on rheumatoid arthritis patients indicated a negative correlation between irisin and RF, ACCP, GPT, GOT, ALP, GPT/GOT ratio, IL-6, CRP, and ESR. as is clearly visible in Table 3.9 [36].
| Parameters | Negative Correlation | No. |
|---|---|---|
| RF | -0.599** | 100 |
| ACCP | -0.263** | 100 |
| GPT | -0.367** | 100 |
| GOT | -0.198* | 100 |
| ALP | -0.583** | 100 |
| IL-6 | -0.603** | 100 |
| CRP | -0.568** | 100 |
| ESR | -0.692** | 100 |
{R=0= No correlation, 0.00< R <0.25= weak extreme, 0.25 < R < 0.75= moderate extreme, 0.75 < R < 1= strong extreme, R=1= perfect, Negative value= inversely}.
**. Correlation is significant at the 0.01 level.
* . Correlation is significant at the 0.05 level.
Table 3.9: Correlation between Irisin and other parameters in RA patients.
Discussion
Immunological Parameters
Rheumatoid Factor (RF) & Anti-Cyclic Citrullinated Peptides (ACCP)
The results of this study confirmed the presence of a significant increase in the concentration of both RF and ACCP in the group of patients compared to the control group. On the other hand, according to the age group, no significant difference was observed when comparing the two groups of patients between them, and this indicates that there is no correlation between age and the concentration of these parameters for patients with rheumatoid arthritis [37]. These results are reasonable and agree with previous studies that indicated that, RF levels were significantly higher in rheumatoid arthritis patients compared with the healthy group. Likewise for, anti-CCP antibody levels were significantly higher in RA than in the healthy group and the combination of the two markers improves diagnostic accuracy, especially in the condition of early rheumatoid arthritis [38].
The reason for the increased concentration of RF in RA patients is likely to be attributed to the increased gene expression of High Mobility Group Box 1 (HMGB1), a nuclear protein that is a biomarker in many active systemic autoimmune diseases such as rheumatoid arthritis. It has been proven that there is a positive correlation between the increase in the level of gene expression of HMGB1 and the levels of RF-IgG.
Furthermore, ACCP concentrations are elevated along with pro-inflammatory cytokines, and ACCP have been shown to contribute to RA pathology either by activating macrophages, activating osteoclasts through immune complex formation, or directly promoting bone loss by binding to Citrulline, vimentin present in bone membranes [39].
Biochemical Parameters
Irisin Level
The results indicate a significant decrease in the concentration of irisin in patients with rheumatoid arthritis compared to the control, and also, when comparing on the basis of age, it was found that the second age group of patients decreased significantly from the first in the concentration of irisin. These results are consistent with the study of Soliman et al., (2020), it was found that serum irisin levels were significantly lower in RA patients compared to controls, On the other hand, the cause of low irisin may be due to lack of sleep, as sleep disturbances are one of the major problems in patients with Rheumatoid Arthritis (RA). To a possible association between low serum irisin with poor sleep in rheumatoid arthritis patients [40].
In this context, someone might ask what is the secret of the link between low irisin and lack of sleep?
The logical explanation for this is that sleep disturbances lead to a decrease in the melatonin hormone, which is usually secreted in the dark during sleep. It has been proven that melatonin increases the level of irisin; therefore its deficiency leads to a decrease in the level of irisin. In addition, the decrease in irisin with aging may be related to the decrease in the concentration of estrogen (E2); it has been shown that there are positive correlations between the level of irisin and estrogen, muscle mass and insulin sensitivity. Conversely, older age and increased fat mass, as well as insulin resistance and high cholesterol, correlate negatively with irisin. The risk of osteoporosis is related to age and gender (increased osteoporosis is observed after menopause), which is partly related to hormonal changes. Estrogen level can affect the synthesis of serum irisin and, in overweight postmenopausal women with osteoporosis, an inverse relationship has been observed between irisin levels and osteoporosis and fractures. On other hand, with age, arthritis patients may develop osteoporosis, which is associated with a deficiency of both calcium and vitamin D, according to studies indicated by Kashat and Ali, 2021. Similarly, the level of irisin in the blood serum is lower in postmenopausal women than in young women, the reason being that the level of E2 is lower due to reduced production of endogenous estrogen. Moreover, E2 decreases during aging which correlates with decreased irisin and loss of muscle mass, Thus E2 may activate the ERα/ERRα axis which can increase FNDC5 expression in skeletal muscle and serum Irisin level [41].
Liver Function Test
The results indicated that there was a significant (p<0.05) increase in the ALP and GPT concentration in the patient group compared to the control group, while there was no significant difference (P>0.05) in the GOT concentration between the two groups. Abnormal liver function has been observed in patients with rheumatoid arthritis. Researchers have also attributed this abnormality to immune disorders and others have justified this by the toxicity of the drugs.
The cause of elevated liver enzymes may be the treatments used in rheumatoid arthritis, as recent study has shown that these treatments improve the outcome and reduce the severity of the disease, but they represent a risk of liver complications. Adverse effects of RA treatments include asymptomatic elevations in liver enzyme, fibrosis, and sometimes fatal hepatic necrosis, On the other hand, liver disorders have been observed in untreated RA patients. Elevated levels of liver aminotransferases"(AST), (ALT) and (ALP)" have been associated with the use of DMARDs in Rheumatoid Arthritis (RA), particularly methotrexate (MTX), Also the elevated liver enzymes in this study may be due to the presence of inflammation, which varies with disease activity, In this context, a positive correlation was observed between the level of ALP and the level of ESR. The analysis also revealed a positive correlation between AST and ALT levels, with CRP level in RA patients.
The results of the activity of liver enzymes showed a significant increase in patients with rheumatoid arthritis compared to the control group, except for AST, the difference was not significant. These results are consistent with another study that indicated an elevated ALT enzyme level in rheumatoid arthritis patients. In addition, other study has reported a slight increase in the level of (AST) in the blood. Moreover, Osteopontin (OPN) is an anti-inflammatory cytokine that induces rheumatoid arthritis, and it is implicated in several liver diseases. On the other hand, Eltahir et al., (2021) concluded that there was no association between Osteopontin (OPN) and GOT, and this may explain the absence of significant differences in GOT concentration between rheumatic patients and the control group in our current study [42].
Inflammatory Parameters
The results showed a significant increase in the inflammatory parameters represented by CRP and ESR. The underlying reason for this increase may be attributed to the decrease in the concentration of irisin, a new protein marker, indicated by Soliman et al, as a predictor of rheumatoid arthritis disease activity and its relationship to cardiovascular risk factors. Serum irisin levels were significantly lower in patients compared to controls. Furthermore, those with high disease activity had the lowest irisin levels. There are significant inverse associations between serum irisin, ESR, and CRP.
These results are in agreement with the results of other studies. It has also been suggested that CRP may be more accurate than ESR in determining RA activity, especially in long-term female patients. ESR was found to be related to the patient's age. The older patient had a higher ESR value which explains the significant increase in ESR value in the second age group of patients compared with the first age group in our current study. Some studies reported that CRP was a better marker of acute disease activity in RA than ESR.
In addition, the reason for the high values of ESR and CRP may be due to the increase in RF and ACCP concentrations confirmed by the results of our current study, and in line with previous study which found that there was a correlation between arthritis and ACCP. and there was a correlation between ACCP and the higher erythrocyte sedimentation rate and C-reactive protein, As well as, there was a correlation between rheumatoid factor and increased Erythrocyte sedimentation rate and C-reactive protein.
On the other hand, the results indicated a significant increase in the concentration of interleukin-6 (IL-6) in patients compared with controls, and the reason for this is likely to be due to the decrease in the concentration of Irisin, as a recent study confirmed that irisin exerts anti-inflammatory effects by modulating the production of several cytokines mainly interleukin-6 (IL-6), IL-1b and tumor necrosis factor-α (Mazur-Bialy et al., 2017).
Interleukin-6 is a cytokine with broad biological activities, involved in a spectrum of age-related diseases such as osteoporosis, It is also an indicator of inflammation within the body, and is produced by Macrophages and monocytes in response to other inflammatory cytokines containing Tumor Necrosis Factor (TNF) beta and interleukin-11. Improvement of IL-6 level observed in ongoing aging and menopausal processes that manifested by osteoclast activation. This explains the increase in the concentration of interleukin-6 in the second age group compared to the first group in light of our findings.
Correlation Analysis
Correlation of Irisin with Immunological Parameters (RF & ACCP)
It is clear from the data analysis that there is a negative correlation between irisin and each of RF, ACCP. To explain this finding, a previous study presented by ALDabbagh and Hashim, which was found that, The values of (RF and ACCP) were also higher in the RA patients than in the controls with a significant difference. These results demonstrate the value of anti-CCP and RF antibodies in predicting the presence of RA.
An increase in these two indicators indicates rheumatoid arthritis; On the other hand, the researchers confirmed that the levels of irisin are low in patients with rheumatoid arthritis. From this point on, the logical relationship between irisin and both RF and ACCP is an inverse correlation. In the "new" criteria, the detection of the cyclic Citrullinated peptide is appropriate to diagnose the disease in an early stage, before joints destructions occur, Cyclic Citrullinated Peptide (CCP) IgG antibodies have been described as highly specific for RA.
Correlation of Irisin with Liver Function Test
Regarding the study of the relationship between irisin and liver enzymes, data analysis confirmed that there is an inverse correlation between irisin and each of the GPT, GOT, and ALP, these results are in line with the findings of Khadim and Al-Fartusie, which confirmed higher concentrations of liver enzymes in patients with rheumatoid arthritis compared to healthy controls. On the other hand, we notice a decrease in the concentration of irisin in patients with rheumatoid arthritis, as we obtain an inverse correlation between irisin and liver enzymes represented by GPT, GOT, ALP in rheumatic patients and this is in line with the findings of Eltahir et al., who confirmed the existence of a slight difference in the concentration of GOT, With a clear significant difference for the GPT concentration between patients and healthy subjects, in line with the study [43].
Correlation of Irisin with Inflammatory Parameters
Data Analysis of our study indicates a negative correlation between irisin and the parameters of inflammation under consideration. To explain this, it must be noted that, the anti-inflammatory property of irisin is associated with decreased phosphorylation and activation of crucial pro-inflammatory cytokines. Recently, irisin treatment reduce the expression of pro-inflammatory factors such as Tumor Necrosis Factor (TNF)-a and Interleukin 6 (IL6). Thus, the decrease in the concentration of irisin is a convincing reason for the high inflammatory indicators in patients with rheumatoid arthritis. Similarly, Afifi et al., observed high values of CRP indicative of active inflammation in RA patients.
On other hand, the study detected significant inverse correlations between serum irisin levels and disease activity measures including ESR and CRP. This was in accordance with others. Furthermore, Study demonstrated that CRP levels were also significantly higher in patients compared to controls. The disease activity score 28 (DAS28) is used to assess the disease course and treatment outcome and is based on a count of 28 specified joints for swelling and tenderness, ESR or C-reactive protein (CRP) which are the disease activity markers [44].
Conclusion
There is a negative association between irisin with aging.
Decreased level of irisin is associated with an increase in autoantibodies RF and ACCP.
The inverse association between irisin and indicators of inflammation gives us a conclusion about the role of irisin as an anti-inflammatory
The increase in liver enzymes due to increasing of inflammation, so it can be concluded that the decrease level of irisin indirectly contributes to the increase in liver enzymes.
Recommendations
We recommend the importance of physical activity and undergoing physical therapy to increase the secretion of irisin and reduce the development of the disease.
Conducting a laboratory study to detect the effectiveness of the use of irisin in the treatment of rheumatoid arthritis and comparing the results with the currently used drugs.
Studying the effect of weight loss on the level of irisin and its relationship to inflammatory parameters in patients.
Conducting a comparative study between patients who depend on physical therapy and others who depend on medications to investigate the effect of the two conditions on liver function.
References
- Afifi N, M Medhat B, Abdel Ghani AM, et al. Value of albumin-fibrinogen ratio and CRP-albumin ratio as predictor marker of disease activity in Egyptian RA patients, correlated with musculoskeletal sonography. Open Access Rheumatol Res Rev 2020; 241-8.
- Al-Dabbagh Ny, Hashim Za. Evaluation of anti-ccp antibodies and rheumatoid factor for the laboratory diagnosis of rheumatoid arthritis. Duhok Medical J 2018; 12:41-54.
- Aletaha D, Smolen JS. Diagnosis and management of rheumatoid arthritis: A review. JAMA 2018; 320:1360-72.
- Alfatlawi R, Al-Mashhadi H, Hameed W, et al. Comparative study between anti-ccp and rheumatoid factor as diagnostic value of rheumatoid arthritis patients. Ann Trop Med Public Health 2019; 23:604-8.
- Alsaedi AA, Al-Ali SJ, Waheed S. The association of the high-mobility group box 1 gene and its product with rheumatoid arthritis in basra province-Iraq. Thi-Qar J Sci 2021; 8:107-12.
- Azadbakht L, Kimiagar M, Mehrabi Y, et al. Soy consumption, markers of inflammation, and endothelial function: a cross-over study in postmenopausal women with the metabolic syndrome. Diabetes Care 2007; 30:967-73.
- Shafransky DR. The effects of restricted environmental stimulation therapy (REST) on serological markers of inflammation in rheumatoid arthritis. Medical College of Ohio 1999.
- Bergmeyer HU, Herder M, Ref R. International Federation of Clinical Chemistry (IFCC). J Clin Chem clin Biochem 1986; 24:497-510.
- Borque L, Barozzi D, Ferrari L. The determination of rhematoid factors by an immunoturbidimetric assay on boehringer mannheim/hitachi analysis systems. Klin Lab 1994; 40:445-53.
- Boström P, Wu J, Jedrychowski MP, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012; 481:463-8.
- Bugatti S, Manzo A, Montecucco C, et al. The clinical value of autoantibodies in rheumatoid arthritis. Front Med 2018; 5:339.
- Coutant F, Miossec P. Evolving concepts of the pathogenesis of rheumatoid arthritis with focus on the early and late stages. Curr Opin Rheumatol 2020; 32:57-63.
- Díaz BB, González DA, Gannar F, et al. Myokines, physical activity, insulin resistance and autoimmune diseases. Immunol Lett 2018; 203:1-5.
- Radovanovic-Dinic B, Tesic-Rajkovic S, Zivkovic V, et al. Clinical connection between rheumatoid arthritis and liver damage. Rheumatol Int 2018; 38:715-24.
- Eda S, Kaufmann J, Roos W, et al. Development of a new microparticle‐enhanced turbidimetric assay for C‐reactive protein with superior features in analytical sensitivity and dynamic range. J Clin Lab Anal 1998; 12:137-44.
- Eltahir MA, Mohammedsalih KA, Mohamed EN, et al. Association between inflammatory cytokines and liver functions in rheumatoid arthritis patients. Sud J Med Sc 2021; 16:276-84.
- Jodeiri Farshbaf M, Alviña K. Multiple roles in neuroprotection for the exercise derived myokine irisin. Front Aging Neurosci 2021; 13:649929.
- Gamal RM, Mohamed ME, Hammam N, et al. Preliminary study of the association of serum irisin levels with poor sleep quality in rheumatoid arthritis patients. Sleep Med 2020; 67:71-6.
- Grootenboer‐Mignot S, Nicaise‐Roland P, Delaunay C, et al. Second generation anti‐cyclic citrullinated peptide (anti‐CCP2) antibodies can replace other anti‐filaggrin antibodies and improve rheumatoid arthritis diagnosis. Scand J Rheumatol 2004; 33:218-20.
- Guo S, Wang R, Jiang T, et al. Alterations and diagnosis potential of serum lipid profiles in rheumatoid arthritis patients. Int J Clin Exp Pathol 2017; 10:3503-9.
- Hanaoka BY, Ithurburn MP, Rigsbee CA, et al. Chronic inflammation in RA: Mediator of skeletal muscle pathology and physical impairment. Arthritis Care Res 2019; 71:173.
- Holers VM, Banda NK. Complement in the initiation and evolution of rheumatoid arthritis. Front immunol 2018; 9:1057.
- Kashat HH, Ali BR. The Role of Some Hormones and Interleukins and Their Relationship with Vitamin D3 Concentration in Osteoporosis Patients. Thi-Qar J Sci 2021; 8:72-6.
- Khadim RM, Al-Fartusie FS. Evaluation of liver function and lipid profiles in Iraqi patients with Rheumatoid Arthritis. J Phys Conf Ser 2021; 1853:012040.
- Korta P, Pochec E, Mazur-Biały A. Irisin as a multifunctional protein: Implications for health and certain diseases. Medicine 2019; 55:485.
- Mahgoub MO, D’Souza C, Al Darmaki RS, et al. An update on the role of irisin in the regulation of endocrine and metabolic functions. Peptides 2018; 104:15-23.
- Mazur-Bialy AI, Bilski J, Pochec E, et al. New insight into the direct anti-inflammatory activity of a myokine irisin against proinflammatory activation of adipocytes: Implication for exercise in obesity. J Physiol Pharmacol 2017; 68:243–51.
- Metzger CE, Anand Narayanan S, Phan PH, et al. Hindlimb unloading causes regional loading-dependent changes in osteocyte inflammatory cytokines that are modulated by exogenous irisin treatment. NPJ Microgravity 2020; 6:28.
- Miyazawa H, Bannai H, Yanase T, et al. A reverse-sandwich enzyme-linked immunosorbent assay for verocytotoxin 1 and 2 antibodies in human and bovine sera. Clin diagn lab immunol 1999; 6:701-4.
- Moore TL, Dorner RW. Rheumatoid factors. Clin Biochem 1993; 26:75-84.
- Palermo A, Strollo R, Maddaloni E, et al. Irisin is associated with osteoporotic fractures independently of bone mineral density, body composition or daily physical activity. Clin Endocrinol 2015; 82:615-9.
- Pandolfi F, Franza L, Carusi V, et al. Interleukin-6 in rheumatoid arthritis. Int J Mol Sci 2020; 21:5238.
- Olago-Rakuomi AA. Prevalence of abnormal liver function tests in rheumatoid arthritis.
- Sauerland U, Becker H, Seidel M, et al. Clinical utility of the anti‐CCP assay: Experiences with 700 patients. Ann N Y Acad Sci 2005; 1050:314-8.
- Schumann G, Klauke R, Canalias F, et al. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37° C. Part 9: Reference procedure for the measurement of catalytic concentration of alkaline phosphatase. Clin Chem Lab Med 2011; 49:1439-46.
- Shi L, Shi L, Wang X, et al. Regulatory roles of osteopontin in production of monocyte-origin MCP-1. Cell Transplant 2018; 27:1185-94.
- Singhal V, Lawson EA, Ackerman KE, et al. Irisin levels are lower in young amenorrheic athletes compared with eumenorrheic athletes and non-athletes and are associated with bone density and strength estimates. PLOS 2014; 9:e100218.
- Soliman S, Gad R, Senosy T, et al. FRI0078 serum irisin level in rheumatoid arthritis patients: Its relationship to disease activity and cardiovascular manifestations.
- Soliman SA, Gad R, Senosy T, et al. Serum irisin level in rheumatoid arthritis patients: Relationship to disease activity, subclinical atherosclerosis, and cardiovascular risk factors. Egypt Rheumatol 2022; 44:109-14.
- Karlsson Sundbaum J, Eriksson N, et al. Methotrexate treatment in rheumatoid arthritis and elevated liver enzymes: A long‐term follow‐up of predictors, surveillance, and outcome in clinical practice. Int J Rheum Dis 2019; 22:1226-32.
- Tanaka H, Kanahashi K, Takekoshi N, et al. Thermoelectric properties of a semicrystalline polymer doped beyond the insulator-to-metal transition by electrolyte gating. Sci Adv 2020; 6:eaay8065.
- Tung YT, Chiang PC, Chen YL, et al. Effects of melatonin on lipid metabolism and circulating irisin in sprague-dawley rats with diet-induced obesity. Molecules 2020; 25:3329.
- Van Delft MA, Huizinga TW. An overview of autoantibodies in rheumatoid arthritis. J Autoimmun 2020; 110:102392.
- Young MF, Valaris S, Wrann CD. A role for FNDC5/Irisin in the beneficial effects of exercise on the brain and in neurodegenerative diseases. Prog Cardiovasc Dis 2019; 62:172-8.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Zainab Qasim Muhammad Al-Yasiri* and Khalid Gatee Al-Fartosi
Department of Biology, College of Science, University of Thi Qar, Nasiriyah, IraqCitation: Zainab Qasim Muhammad Al-Yasiri, Khalid Gatee Al-Fartosi, Correlation of Irisin with Inflammatory Parameters and Liver Function in Rheumatoid Arthritis Patients, J Res Med Dent Sci, 2023, 11(7):01-07.
Received: 24-Jun-2023, Manuscript No. jrmds-23-89450; Accepted: 27-Jun-2023, Pre QC No. jrmds-23-89450; Editor assigned: 27-Jun-2023, Pre QC No. jrmds-23-89450; Reviewed: 11-Jul-2023, QC No. jrmds-23-89450; Revised: 17-Jul-2023, Manuscript No. jrmds-23-89450; Published: 24-Jul-2023
