Research Article - (2021) Volume 9, Issue 12
Correction of the Endothelial Function and the Hemostasis System Disorders with Ademethionin and Taurine in Model Associated with ADMA-Like Preeclampsia
Tatiana G Pokrovskaya1*, Taisiya A Khadieva1, Vladimir V Gureev1, Vladimir M Pokrovskii1, Evgenii A Patrachanov1, Igor B Kovalenko1, Vladimir I Shutov1 and Alexey A Shabalin2
*Correspondence: Tatiana G Pokrovskaya, Department of Medical and Dental Science, Belgorod State National Research University, Belgorod, Russia, Email:
Abstract
Objective: The aim of this study is to evaluate the effectiveness of pharmacological correction of functional disorders of endothelial function and the hemostasis system with ademethionin and taurine in ADMA-like preeclampsia model as a serious problem of modern medicine.
Materials and methods: Simulation of ADMA-like preeclampsia was performed by intraperitoneal injection of L-NAME to females in the same doses for 7 days (14-20th days of pregnancy). Deficiency of nitric oxide in result of the blockade of NOsynthase was accompanied by violation of endothelium dependent and endothelium undependentvasodilation assessed in pharmacological trials, which was reflected in the increase of the coefficient endotelialny dysfunction. The platelet aggregation induced by ADP, collagen, ristomycin, adrenaline was determined, as well as PTT, TT, aPTT, fibrinogen, and the clotting time. The animals were divided into the following groups: Intact – pregnant + 0.9% NaCl from the 14th to the 20th days of pregnancy. (n=10); -NAME) – pregnant + ADMA-like agent (with intraperitoneal administration of L-NAME at a dose of 25 mg/kg once a day from the 14th to the 20th days of pregnancy) (n=10); Pregnant + L-NAME 25 mg/kg + Taurine (50 mg/kg/day orally) (n=10); Pregnant + L-NAME 25 mg/kg + Taurine (50 mg/kg/day orally) (n=10); Pregnant + L-NAME 25 mg/kg +Ademethionine (150 mg/kg/ day orally (n=10); Pregnant + L-NAME 25 mg/kg + Taurine (50 mg/kg/day orally) +Ademethionine (150 mg/kg/ day orally (n=10).
Results: Introduction of ademethionin and taurine resulted in a decrease in thrombocyte aggregation capacity from 53.8±2.60% to 27.7±1.25% when using ADP as an inducer. The clotting time was from 841±42 s up to 1152±25 s. In addition, there was an increase in temporal parameters of plasma-coagulation hemostasis and a decrease in plasma fibrinogen content. In the group with use of taurine the coefficient of endothelial dysfunction decreased to the level of intact animals. Use of ademethionine and taurine combined resulted in the most pronounced endothelioprotective effect on the ADMA-like preeclampsia model. The coefficient of endothelial dysfunction decreased more than when using monotherapy of these drugs.
Conclusion: In animals with experimental preeclampsia, there were disturbances in the hemostasis system, comparable to those in the clinical situation. The use of ademethionine and taurine leads to a pronounced correction of hemostasis parameters. Possibly, ademethionine and taurine have an endothelioprotective effect because of their ability to decrease hyperhomocysteinemia.
Keywords
Experimental preeclampsia, platelet aggregation, hemostasis, endothelial dysfunction, ademethionine, taurine, homocysteineIntroduction
Endothelial Dysfunction (ED) is a pathological state of the endothelium. The basis of its pathogenesis is violation of the synthesis of vasoactive substances. One of the main vasoactive substances secreted by endotheliocytes is nitrogen oxide (NO), an endothelial vasodilator [1]. The impaired synthesis of NO results in changes of the vasoregulating function of the endothelium, followed by vasoconstriction. At the end of complex pathogeneticprocesses of the response with the release of a large number of humoral factors are an increase in the content of ADMA (asymmetric dimethylarginin), endothelial dysfunction, secondary ischemic lesions, impaired hemostasis and oxidative stress [2]. The hemostasis system includes a vascular-thrombocytic unit, which consists of endothelium and platelets, and a plasmacoagulation unit, which consists of the blood coagulation system, the blood anticoagulation system and the fibrinolytic system. Simulation of preeclampsia in the experiment and its correction are also accompanied by a disturbed platelet aggregation ability and its normalization, respectively. There have only been a small number of scientific publications written recently which discuss a certain independent role of platelets in the development of preeclampsia and the possibility of preventing and treating placental-mediated complications with drugs correcting the function of platelets. Oxidative stress leads to ED, as oxygen free radicals are actively involved in reducing nitrogen oxide. Reducing the time of a direct effect of nitric oxide on target cells also leads to the development of endothelial dysfunction. Due to the main role of oxidative stress in ED development, LOP products and antioxidant defense indicators can be the markers of ED.
During physiological pregnancy, the balance of production of vasoactive factors is shifted by endothelium towards maintaining vasodilation, due to the constant release of basal nitric oxide, which ensures adequate perfusion of placental tissue. Nitrogen oxide, being a potent vasodilator factor, is formed from Larginine under the action of the endothelial NO synthase enzyme (eNOS). The blood accumulation of methylated Larginine analogues –ADMA (asymmetric dimethylarginine) and MMA (NG-monomethyl-Larginine, which are endogenous inhibitors of endothelial NO-synthase (eNOS) – leads to impaired vasodilation and causes preeclampsia (Speer et al. 2008). As known, ADMA is formed as a result of the catabolism of proteins with residues of methylated arginine under the influence of protein arginine methyltransferase (PRMT). Even a slight concentration of ADMA is enough to suppress the activity of NO synthase (NOS).
Increased homocysteine is known to be a predictor of pre-eclampsia in pregnant women and a risk factor for cardiovascular diseases. Hyper homo cysteinemia can both exacerbate existing damage to the endothelium and be an independent factor causing the development of endothelial dysfunction.
Studies have shown that women are highly likely to develop cardiovascular diseases in future if they have had pre-eclampsia, gestational diabetes and early labor. These pathophysiological conditions are caused by ED, the ensuing vasoconstriction and a reduced placental, renal and cerebral blood flows. Moreover, impaired blood flow in the uterine arteries can lead to the retarded growth of the fetus, hypamnion and placental abruption. Due to the involvement of NO in the ED pathogenesis, its reduced synthesis and increased biodegradation resulting from oxidative stress, a search for additional pharmacological correction of ED as a predictor of cardiovascular pathology and preeclampsia is viewed as urgent. The endothelium protective effects of ademethionine and taurine are being actively studied. Taurine and ademetionin are known to effectively prevent the development of endothelial dysfunction, ischemic damage to organs and systems. Taurine has an antioxidant effect, restores the expression of endothelial NO synthase, which is the main endothelium protective pharmacodynamic effect. Ademethionine is involved in transmethylation, transsulfurization, and transamination reactions, and by inhibiting the PRMT enzyme and preventing the ADMA formation, leads to an increased expression of the eNOS enzyme. In addition, when they were used, a pronounced effect was observed when correcting oxidant disorders under conditions of ADMAlike preeclampsia [3].
Materials and Methods
The work was organized and carried out in compliance with the following regulatory acts and guidelines governing the conduct of experimental research in the Russian Federation:
• Order of the Ministry of Healthcare of the Russia of April 1, 2016 No. 199N ”On Approval of the Rules of Good Laboratory Practice” (Order of the Ministry of Healthcare of the Russia 2016).
• GOST 33044-2014 “Principles of Good Laboratory Practice” (GOST 2015).
• GOST 33217-2014 “Guidelines for the Maintenance and Care of Laboratory Animals. Rules for the Maintenance and Care of Laboratory Predatory Mammals” (GOST 2016).
• “Guidelines for Conducting Preclinical Studies of New Drugs”, Ed. Mironov A.N., - M.: Grif and Co. – 2012.
• The ethical principles of treating laboratory animals were in compliance with “The European Convention for the Protection of Vertebral Animals Used for Experience and Other Scientific Purposes. CETS No. 123”.
The experiments were performed on 50 Wistar female rats weighing 170-240 g. To form groups of pregnant animals with predetermined periods, the males (2 animals) were introduced to the females, which had been kept separately, for 24 hours. Then the animals were separated, and 10 days later, during the ether sleep, pregnancy was determined by palpation. In the experiments, pregnancy occurred in 30-40% of cases. ADMA-like agent – non-selective blocker NO-synthase Nnitro- L-arginine-methyl ether (L-NAME) – was administered intraperitoneally at a dose of 25 mg/kg/day for seven days (14-20th days of pregnancy) (Gureev et al. 2014).
Then the animals were divided into the following groups:
• intact (n=10);
• Preeclampsia (n=10);
• Preeclampsia+ademethionine 150 mg/kg (n=10);
• Preeclampsia+taurine 260 mg/kg (n=10);
• Preeclampsia+ademethionine 150 mg/kg+taurine 260 mg/kg (n=10).
Ademethionine and taurine, as well as their combination, were injected to the experimental animals intragastrically daily (through an atraumatic probe) at a dose of 150 mg/kg/day and 260 mg/kg/day against the injection of L-NAME to them. The combination of ademethionine and tau-rine was injected at the same doses, via the same routes of administration and the same duration of therapy. Taurine was administered one hour after the administration of ademethionine.
Functional Vascular Tests On the 8th day of the experiment (the 21st day of pregnancy), a catheter was inserted into the left carotid artery of the animals to record hemodynamic parame-ters. The animals were at that moment under anesthesia. Bolus dosing of vascular and cardiac samples was in the right femoral vein. The hemodynamic parameters, namely, Systolic Blood Pressure (SBP), Diastolic Blood Pressure (DBP), and Heart Rate (HR) were measured continuously by means of a TSD104A sensor and a Biopac MP150 hardwaresoftware complex. Acetylcholine (40 µg/kg) was injected intravenously to determine endothelium-dependent vasodilatation , and sodium nitroprusside (30 µg/kg) was injected intravenously to determine endotheli-umindependent vasodilation.
The Coefficient of Endothelial Dysfunction (CED) was calculated as the ratio of the area of the triangle above the blood pressure recovery curve in the endotheliumindependent vaso-dilation reaction to the area of the triangle above the BP recovery curve in the endotheliumdependent vasodilation reaction in terms of mean arterial pressure.
Biochemical assessment of endothelial function. The total nitrates and nitrites were measured in one step by using a modified method; the concentration of homocysteine was determined by an enzymatic method using a Pliva- Lachema Diagnostika s.r.o set. The content of Lipid Peroxidation Products (L??) in the blood plasma was also determined: diene conjugates (DC, relative units/ ml), malonic dialdehyde (MDA, µmol/l), oxidized lowdensity lipoproteins (OxLDLs, µmol/L); the superoxide dismutase level (SOD, relative units/ml) and the total antioxidant activity of blood serum (???, %) were also studied. The data obtained were statistically processed using Statistica 10.0 software. Descriptive statistics applied to all data. The normality of distribution was assessed by means of Shapiro-Wilk and Kolmagorov- Smirnov tests. Statistical significance depending on the particular data was assessed by using the criteria of Student and Mann-Whitney tests with the Bonferroni amendment. Differences at p<0.05 were recognized as statistically significant. Determination of coagulation indicators and a degree of thrombocyte aggregation was performed on the 21st day of gestation. Blood from the abdominal aorta was collected into a test tube with a 3.8% solution of sodium citrate in a ratio of 9:1, followed by centrifugation of 1000 rpm for 10 minutes. Antiplatelet activity was determined by G.Born’s method modified by Z.A on a two-channel laser analyzer of platelet aggregation ALAT-2 (Biola). ADF (at a final concentration of 5 µM), collagen (50 µg/ml), ristomycin (5 µM), adrenaline (10 µM) were used as inducers (manufactured by SPD RENAM, Russia). Analysis was performed no later than 2 hours after obtaining blood. Coagulation indicators were determined on a Start 4 (Diagnostica Stago, France) analyzer using reagents for the determination of prothrombin time, thrombin time, activated partial thromboplastin time (aPTT), fibrinogen test (manu-factured by SIEMENS). Thrombus formation time was determined on the 20th day of pregnancy in anesthetized (300 mg/kg of chloral hydrate) female rats with experimental preeclampsia. Thrombus formation was caused by applying a 50% solution of iron (III) chloride, for which an area of the exposed carotid artery was isolated from the surrounding tissues, and a cotton pad moistened with a 50% solution of iron chloride (0.025 ml) was placed on it. The blood flow was recorded above the site of application, using a Doppler probe (Minimax-Doppler-K, St. Peters-burg). The time of blood clot formation from the moment of application of the iron (III) chloride solution to the complete cessation of blood flow in the carotid artery was noted [4].
Results and Discussion
In the simulation of ADMA-like preeclampsia, the use of ademe-thionine 150 mg/kg and taurine 260 mg/kg in monotherapy, unlike the combined use, was found to restore BP to the baseline values that did not significantly differ from those in intact animals (Table. 1).
| Groups | Functional test | SBP | DBP | HR | S | CED |
|---|---|---|---|---|---|---|
| Intact | Initial | 128.1 ± 5.2* | 90.3±4.8* | 453.8 ± 11.6 | - | 1.4 ± 0.1* |
| AH | 59.3 ± 2.0* | 32.4±1.6* | 448.3 ± 11.0 | 1256.2 ± 219.3 | ||
| NP | 77.8 ± 5.9* | 47.7±3.4 | 464.1 ± 11.5 | 1207.2 ± 77.0* | ||
| L-NAME | Initial | 180.0 ± 8.9 | 131.0±6.4 | 417.2 ± 11.7 | - | 4.1 ± 0.4 |
| AH | 113.5 ± 3.6 | 75.3 ± 2.4 | 399.8 ± 15.6 | 1204.8 ± 130.6 | ||
| NP | 104.7 ± 3.5 | 50.6 ± 2.9 | 401.7 ± 11.8 | 3480.1 ± 329.9 | ||
| L-NAME + ademetionine | Initial | 158.6±4.7 | 137.4±4.3 | 385.9 ± 15.8 | - | 1.8 ± 0.2* |
| AH | 95.4±6.2*y | 73.8±7.5y | 371.3±14.0 | 1240.8±177.2 | ||
| NP | 101.8±8.4y | 83.6±8.3*y | 396.2±24.5 | 2004.0±208.6* | ||
| L-NAME + taurine | Initial | 134.2±4.9*? | 104.2±7.7*? | 385.9±15.8 | - | 1.5±0.4* |
| AH | 75.9±3.1*? | 61.4±4.3* | 371.3±14.0 | 1312.9±186.2 | ||
| NP | 56.5±3.2*? | 41.1±2.5*? | 396.2±24.5 | 1650.3±255.9*? | ||
| L-NAME + ademetionine + taurine | Initial | 123.5±3.6* | 106.2±4.5* | 331.6±31.4* | - | 1.4±0.2* |
| AH | 78.6±4.5* | 52.3±2.4* | 335±36.4 | 1370.1±220.4 | ||
| NP | 73.1±3.5* | 51.0±3.2 | 331.4±14.2 | 1680.3±322.3* |
Note: SBP - systolic blood pressure; DBP - diastolic blood pressure; HR - heart rate; S – area above the vascular vasodilation response curve; CED – coefficient endothelial dysfunction (the ratio of the areas of vasodilation to the administration of nitroprusside and acetylcholine) * - p <0.05 in comparison with a group of animals with L-NAME animals; y - p <0.05 - in comparison with a group of animals that received a combination of ademetionine 150 mg / kg and taurine 260 mg. The combined use of ademetionine (150 mg/kg) and taurine (260 mg/kg) had a pronounced endothelioprotective effect, with ?ED decreasing to 1.4±0.2. These indicators were better than when using the monotherapy with ademetionine (1,8±0,2) or taurine (1,5±0,4) (Table. 1).The concentration of nitrite ions (NOx) in pregnant female rats with ADMA-like preeclampsia is 48,2±4,0 µM, and in intact animals –118,3 ±4,0 µM. The combined use of ademetionine 150 mg/kg with taurine 260 mg/kg increases the content of nitrite ions to 80,0±4,2 (Table 2).The use of ademetionine and taurine at these doses also had a positive effect on the indices of lipid peroxidation and the antioxidant system (Table 2).
Table2: Effect of Ademetionine, Taurine and Their Combination on Lipid Peroxidation, Antioxidant System in Pregnant Rats when Simulating ADMA-like preeclampsia (L-NAME-induced Preeclampsia (L-NAME-PE)) (M±m).
| Indicator | Intact | L-NAME- preeclampsia | L-NAME- preeclampsia + ademethionine 150 mg/kg | L-NAME- preeclampsia + taurine 260 mg/kg | L-NAME- preeclampsia + ademethionine 150 mg/kg + taurine 260 mg/kg |
|---|---|---|---|---|---|
| NOx, mkmol/l | 118.3 ± 4.0* | 48.2 ± 4.0 | 67.1 ± 3.8* | 72.5 ± 3.7* | 80 ± 4.2* |
| DC (relative units/ml) | 0.11 ± 0.01* | 0.38 ± 0.02 | 0.25 ± 0.02* | 0.20 ± 0.01* | 0.18 ± 0.01* |
| MD? (mkmol/l) | 0.34 ± 0.02* | 1.63 ± 0.02 | 1.30 ± 0.16* | 1.02 ± 0,02* | 0.90 ± 0.02* |
| OxLDL (mkmol/l) | 0.03 ± 0.01* | 0.16 ± 0.02 | 0.15 ± 0.02* | 0.10 ± 0.01* | 0.08 ± 0.01* |
| SOD (relative units/ml) | 15.20±0.38* | 13.00± 0.29 | 14.35± 0.19* | 14.61 ± 0.22* | 15.18 ± 0.19* |
| T?? (%) | 41.62±0.80* | 35.40± 0.80 | 38.62 ± 1.30 | 40.01 ± 0.39 | 40.10 ± 0.40* |
When simulating ADMA-like preeclampsia, a significant increase in the level of homocysteine in the blood of pregnant females was observed, compared to that in the intact ones – 6.5 ± 1.4 and 5.0 ± 1.2 µmol/l, respectively.
When correcting PE with the combination of ademethionine 150 mg/kg and taurine 260 mg/kg, a significant decrease in the level of homocysteine was observed to 4.9 ± 1.3 µmol/l (Figure 1).
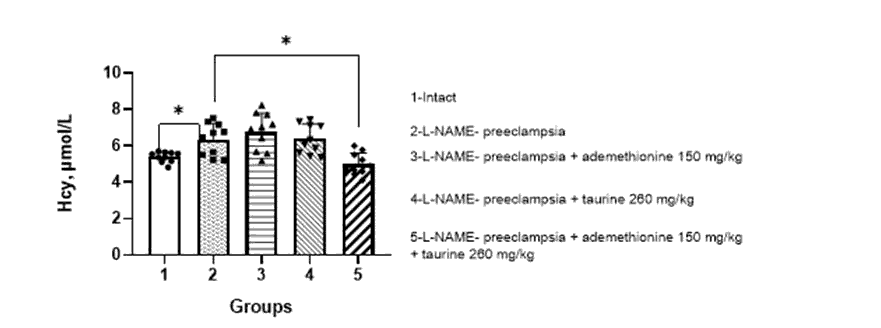
Figure 1: Effect of ademethionine, taurine on the level of homocysteine in the blood of pregnant rats against the background of simulated ADMA-like preeclampsia (M±m).
Note: Hcy – homocysteine. * – p<0.05 compared with pregnant rats with ADMA-like preeclampsia/
Modeling ADMA-like preeclampsia by intraperitoneal administration of L-NAME at a dose of 25 mg/kg/day resulted in an increase in platelet aggregation, which is evidenced by an increase in the maximum plasma light transmission (Table 3). When using ADF as an inducer, the light transmission increased from 24.3±1.09% to 53.8±2.60%, when using collagen – from 50.2±1.66% to 81.3±2.68%, when using ristomycin – from 71.8±1.65% to 85.7±0.80% and when using adrenaline – from 30.1±1.97% to 52.6±2.23%. When administering the studied drugs, ademetionine and taurine, to the animals with ADMA-like preeclampsia, aggregation of platelets with individual peculiarities was corrected. (Table 3). When induced with ADP, collagen and adrenaline against the use of taurine, there was a statistically significant decrease in platelet aggregation relative to that in the control (p<0.05), but it did not reach the target level and was statistically different from that in the group of intact animals (p<0.05). When inducing with ristomycin, the platelet aggregation level was statistically lower (p<0.05) than that of the ”untreated” animals and reached the target level, as evidenced by there being no statistical difference from the platelet aggregation level of the animals of the intact group (Table 3).
Table3: The effect of ademethionin and taurine on the time of induced platelet aggregation in modeling ADMAlike preeclampsia (M ± m; N = 10).
| Group | ADP | Collagen | Ristomycin | Adrenaline |
|---|---|---|---|---|
| Intact | 24.3±1.1%* | 50.2±1.66%* | 71.8±1.65%* | 30.1±1.97%* |
| L-NAME | 53.8±2.6%y | 81.3±2.59%y | 85.7±0.80%y | 52.6±2.23%y |
| Ademethionin | 54.8±3.2%* | 65.3±2.38%*y | 81.2±1.13%* | 46.7±1.96*y |
| Taurine | 51.5±1.4%* | 40.2±2.15%*y | 61.2±1.76%*y | 22.8±1.89%*y |
| Ademethionin + Taurine | 27.7±1.7%*y | 53.6±2.23%*y | 62.8±2.12%*y | 21.7±1.0* |
With the development of preeclampsia, events occur in different conditions. It should be noted that platelet activation can be caused not only by proteins of subendothelial structures, but also by adrenaline, norepinephrine, ADP, immune complexes, peroxide radicals, hypoxia, arterial hypertension, prostaglandins PgG2 and PgH2, arachidonic acid, thromboxane A2, platelet activating factor (PAF), phorbol esters, latex, lectins, A23187 ionophore, bacteria and bacterial lipopolysaccharide, tumor cells, increased shear stress, etc. [9, 10, 11]. It is ischemic placenta that excretes a large number of these humoral factors, which leads to platelet activation. Depending on the inducers and their combination, the activation of platelets, as well as the humoral factors secreted by them and their ratios are of a different nature. Unlike natural conditions, there is no exposed subendothelial layer, nor is there platelet adhesion at the site of activation. The mass of activated platelets goes into the systemic circulation, and it can be said that there is a kind of endocrine gland, located throughout the circulating blood and directly contacting with endothelium. Therefore, even if the action of each individual platelet is considered locally, their mass location determines the generalization of their effects. When passing through the ischemic placenta, more and more new platelets are activated. When a critical number is reached, the level of humoral factors secreted by platelets becomes sufficient to cause systemic endotheliosis.
There is even a parallel between the DIC-syndrome, systemic inflammatory response syndrome and preeclampsia. One of the diagnostic criteria for the severity of preeclampsia is the sFlt-1/PlGF ratio. sFlt-1 is a variant of the vascular endothelial growth factor receptor (VEGFR-1). The higher this ratio, the higher the risk of preeclampsia or its severity. The source of sFlt-1 is not only an ischemic placenta, but also a plateletmonocine plateletmonocine complex, which is obtained because of aggregation of platelet-activated monocyte.
During pregnancy, platelet sensitivity to adrenaline, collagen, ristomycin, and other factors increases. This accurately determines polyetiology and another point of application of provoking factors in the pathogenesis of preeclampsia in such conditions as: infections, psychoemotional stress, conditions accompanied by excessive formation of peroxide radicals, metabolic syndrome, conditions accompanied by an increased content of ADMA and homocysteine, etc.
Thus, platelet activation is an intermediate pathogenetic link between the immaturity of the spiral arteries, ischemia of the placenta and systemic endothelial dysfunction. The chain of events described above looks logical in other pathological conditions as well, such as: sepsis, atherosclerosis, arterial hypertension, metabolic syndrome, varicose veins, etc. Summarizing the analyzed data, it can be said with a certain degree of accuracy that vascular-coagulation-immunological disorders in pregnant women have a common pathogenetic interaction in various diseases and, depending on the provoking factors and individual characteristics, determine the development of DIC-syndrome, preeclampsia or eclampsia [5].
An increase in platelet aggregation capacity and activation of the plasma-coagulation component of hemostasis, oxidative stress and mitochondrial dysfunction in modeling ADMA-like preeclampsia repeat the patterns observed in the clinic in women with preeclampsia. L-NAME is an analogue of ADMA, which, in turn, is an eNOS inhibitor [18, 19, 20, 21, 22]. That is why the main task of pharmacology is the search and study of active pharmacological agents aimed at the prevention [23, 24] and correction [25, 26, 27] of ischemic processes occurring in various organs and tissues. the search for new substances and ways to increase the resistance of tissues to ischemia remains relevant, despite the rapid development of experimental pharmacology [28, 29, 30, 31], the spread of peptide drugs [32, 33, 34, 35, 36], the improvement of targeted synthesis methods that allow the creation of highly selective drugs [37, 38, 39].
Therefore, the introduction of L-NAME results in a reduced activity of eNOS ,, as well as a reduced formation of potent antiaggregants – NO – by endothelium. At this stage, the most sensitive are the placental vessels. The resulting endothelial dysfunction leads to ischemic events in it. When passing through the placenta, platelets get activated due to ischemia, as well as due to humoral factors released in response. Activated platelets not only have an increased aggregation capacity by themselves, but also activate intact platelets, secreting aggregation inducers into the bloodstream. Modeling ADMA-like preeclampsia leads to a pronounced persistent increase in blood pressure. There is a change in shear rate, which can also activate platelets. According to the literature, in women with preeclampsia, on the surface of the platelet, the expression of receptors to different aggregation inducers increases compared with that in normal pregnancy. This contributes to increased sensitivity of platelets in them. No concrete literature data on the number of receptors for aggregation inducers in animals with ADMA-like preeclampsia have been found, but taking into account the comparable pathogenetic mechanisms of both pathological conditions, the probability of this mechanism is rather high.
The time of the blood clot formation until the complete cessation of blood flow in the artery is an integral sum of vascular-platelet and plasma-coagulation components of hemostasis. Therefore, its shortening is a logical consequence of a shift of hemostasis towards hypercoagulation. The pronounced positive effects of ademetionine in the correction of platelet aggregation disorders caused by the ADMA-like preeclampsia model are accounted for by several direct and indirect mechanisms. The literature describes various positive effects of ademetionine: improvement of mitochondrial respiration, increased the intraplatelet concentration of glutathione, enhanced the antiaggregant effect both red blood cells and leukocytes of ademetionine (SAMe), as did simultaneous incubation with L-arginine [40], antiiinflammatory effects by changing the concentration of interleukins: the content of tumor necrosis factor (TNF) a decreases and the synthesis of interleukin-10 (IL-10) is activated. In addition, the ratio of SAM to SAH (S-adenosyl homocysteine) changesalso was by SAMe [41].
The study of the effect of SAM on the expression of the antioxidant stress proteins heme oxygenase-1 and ferritin in endothelial cells showed that the induction of the oxygenase / ferritin system leads to the protection of tissues from inflammatory factors. Ademetionine increases the levels of mRNA and oxygenase in endothelial cells. The induction of oxygenase gene expression is associated with an increase in ferritin levels and is regulated at the transcriptional level through an increase in promoter activity. The activation of oxygenase under the influence of SAM is associated with a decrease in the NADP-mediated release of reactive oxygen species. Thus, the HO-1 / ferritin system can be another target for the antioxidant action of ademetionine .Ademetionine is able to prevent the toxic effects of xenobiotics in various tissues. It was shown that its use improved the growth of animal embryos, reducing the embryotic effect of ethanol. Ademetionine does not possess embryophetotoxicity, does not have any negative effect on reproductive function, and does not cause any developmental abnormalities [44]. It not only eliminates the symptoms of cholestasis in the mother and prevents possible complications of pregnancy, but also reduces the incidence of premature birth in patients with cholestasis, neu-tralizes the toxic embryopathic effects of xenobiotics and maintains the normal functioning of the placenta. Direct infusion of ademenionine (S-adenosylmethionine, AdoMet) to the brain cortex of thioacetamide (TAA) rats decreased extracellular concentration of dimethylarginines. The possible explanation of this phenomenon is associated with a feedback inhibition of PRMT by S-adenosylhomocysteine (AdoHcy), formed from AdoMet upon trans-methylation [45, 46]. The intensity of this inhibition was reflected by the diminished dimethylarginine levels, the feedback inhibition of dimethylarginines (DMAs) synthesizing enzymes (PRMTs). PRMT enzyme by AdoHcy seems the most reliable explanation of the observed decrease in asymmetric dimethylarginine (ADMA) concentration. Increased circulating ADMA levels, which inhibits NO synthesis, may be associated primarily with endothelial dysfunction that somehow can be translated on changes of CBF considered as a causative and a predictive factor of overt hepatic encephalopathy (HE). This study reveals the ability of ademetionine to directly block the formation of asymmetric dimethylarginine. DNA methylation is altered in preeclampsia placentas determined the methylation index by measuring placental S-adenosylmethionine (SAM) and Sadenosylhomocysteine (SAH) levels hypomethylation state of the placenta in early onset preeclampsia (EOPE), which is reflected by lower SAM and placental growth factor (PlGF) DNA hypomethylation underlines the possible role of placental DNA hypomethylation in the pathophysiology of EOPE, which needs further investigation the use of ademetionine in correction EOPE.
Taurine appears to have a protective effect against the hyper-coagulative state in edema, proteinuria and hypertension gestosis. Plasma and platelet taurine levels are reduced in patients with type 1 diabetes mellitus. The pronounced positive effects of taurine in the correction of platelet aggregation disorders caused by an ADMA-like model of preeclampsia are also associated with direct and mediated mechanisms. The literature also describes the positive effects of taurine in pregnant women in particular, its ability to reduce platelet aggregation, including not through the Ca2+-dependent mechanism. Summing up, it should be noted that the proposed methodological complex of functional, biochemical and morphological changes linked with the development of NO deficiency due to the blockade of NO-synthase in male rats and pregnant female rats against the background of preeclampsia makes it possible to quite objectively detect and evaluate the endotheliotropic effect of pharmacological agents.Thus, the most pronounced endothelioprotective effect on the model of ADMA-like preeclampsia was observed with the combined use of ademethionine and taurine, which was reflected in a decreased CED.The maximum reduction in ?ED to the level of that in the intact animals, against the background of simulated L-NAME-induced NO deficiency in male rats, was recorded when using taurine 260 mg/kg and amounted to 1.3 ± 0.1.
It should be noted that the introduction of the studied drugs at the studied doses showed their endothelium and cardioprotective activity, but was not accompanied by reaching the target blood pressure indicators. This, on the one hand, indicates that ?ED has independent significance, and, on the other hand, that L-NAMEinduced arterial hypertension on the 7th day involves into the pathogenic process not only the nitroergic system, but also all the elements of the humoral and neurogenic regulation contours of the circulatory system. The study of the NO-producing function of the endothelium confirms the endothelioprotective effects of ademethionine, taurine, and their combination, which shows in preventing the reduction of stable metabolites of nitric oxide under conditions of endothelial dysfunction. On the model of ADMA-like preeclampsia in pregnant rats, the maximum reduction in ?ED was observed when using ademethionine 150 mg/kg and taurine 260 mg/kg. The level of homocysteine, which is a marker of preeclampsia, also decreased to the level of that in the intact animals with the combined use of ademethionine 150 mg/kg and taurine 260 mg/kg in the ADMA-like preeclampsia model. Therefore, the combination of ademethionine with taurine on the model of ADMA-like preeclampsia can be one of the rational approaches for correcting endothelial dysfunction during pregnancy in clinical practice, since the effect of this combination on the nitroergic and neurohumoral systems is pathogenetically justified, and these drugs are approved for use in pregnant women. The way of how damage to the system is caused by L-NAME, as well as points of application of ademethionine, which increases the amount of NO, and taurine, which simulates the synthesis of NO, due to capturing reactive oxygen species. From the literature it is known that ademethionine has an endothelioprotective effect by blocking the protein arginine methyltransferase (P?MT) enzyme – an enzyme that participates in the synthesis of asymmetric dimethylarginine (ADMA), and taurine, in turn, is a powerful antioxidant: it inhibits the production of superoxide radicals, decreases the production of the factor, and decreases the tumor necrosis factor, which is a marker of placenta inflammation, and restores the expression of endothelial NO synthase
SAM or ademethionine is formed endogenously from methionine amino acid using the methionine adenosyl transferase enzyme. Further, S-adenosylhomocysteine is formed from SAM under the action of SAM-dependent methyltransferase enzyme, and it is hydrolyzed to homocysteine by S-adenosylhomocysteine hydrolase. Homocysteine, completing the cycle, is converted to methionine in the transfer reaction of the methyl group from 5-methyltetrahydrofolate, one of the two classes of methionine synthases. With a hereditary defect of enzymes involved in the SAM cycle and with an excessive intake of ademethionine, remethylation processes can be disturbed, which leads to an accumulation of homocysteine in the blood, i.e. hyperhomocysteinemia, which results in oxidative stress and ED.
However, in this L-NAME model of induced endothelial dysfunction, the administration of ademethionine had endothelium protective effects and reduced oxidative stress. This may have happened because most of the administered ademethionine inhibited PRMT, which contributed to an increase in NO. And as known, one of the main vasoactive substances secreted by endotheliocytes is nitric oxide –an endothelial vasodilator.
Taurine is a sulfur-containing amino acid, which is synthesized in the body from cysteine, which is formed from homocysteine with the participation of pyridoxal phosphate. Thus, therapy with ademethionine can be used in case of pathologies with pronounced oxidative stress. It can also be combined with a drug with antioxidant properties – taurine.
The combinatione effects of ademethionine and taurine is explained by the ability to enhance the positive effects of the investigated pharmacological agents in the correction of endothelial dysfunction disorders and hemostasis disorder sin animals with ADMA-like preeclampsia and, thus, to a greater extent lead to a decrease in the number of factors contributing to the transition of platelets into the activated form.
Conclusion
Ademethionin at a dose of 150 mg/kg and taurine at a dose of 260 mg/kg showed an endothelium protective effect on ADMA-like preeclampsia model both in monotherapy and in combination. On the model of ADMAlike preeclampsia in pregnant rats, the maximum reduction in ?ED was observed when using ademethionine 150 mg/kg and taurine 260 mg/kg. The level of homocysteine, which is a marker of preeclampsia, also decreased to the level of that in the intact animals with the combined use of ademethionine 150 mg/kg and taurine 260 mg/kg in the ADMA-like preeclampsia model. When modeling ADMA-like preeclampsia, there is an increase in platelet aggregation from about 19% to 121% when the inducers used are ADP, collagen, ristomycin and adrenaline, as well as a shift in the plasma-coagulation component of hemostasis towards hypercoagulation. The time of blood clot formation was reduced to 70% of the original. The use of ademethionin and taurine resulted in a pronounced correction of the endotyelial dysfunction, disturbance of induced platelet aggregation, indicators of the plasma-coagulation component of hemostasis and the time of thrombus formation in animals with ADMA-like preeclampsia.
References
- Félétou ?, Vanhoutte PM. Endothelial dysfunction: a multifaceted disorder (The Wiggers Award Lecture). Am J Physiol Heart Circ Physiol 2006; 291: H985-H1002.
- Franchi AM, Barani MA, Correa F, et al. Resveratrol protects from lipopolysaccharide-induced inflammation in the uterus and prevents experimental preterm birth. Mol Hum Reprod 2017; 23: 571-581
- Tyurenkov IN, Perfilova VN, Karamysheva VI, et al. Effect of GABA derivatives on the rate of thrombus formation, platelet aggregation, and plasma coagulation capacity in rats with experimental gestosis. Bull Exp Biol Med 2014;158: 219-221
- Major HD, Campbell RA, Silver RM, et al. Synthesis of sFlt-1 by platelet-monocyte aggregates contributes to the pathogenesis of preeclampsia. Am J Obstet Gynecol 2014; 210: 547.
- Bergen NE, Jaddoe VW, Timmermans S, et al. Homocysteine and folate concentrations in early pregnancy and the risk of adverse pregnancy outcomes: the Generation R Study. BJOG 2012;119:739–751.
Author Info
Tatiana G Pokrovskaya1*, Taisiya A Khadieva1, Vladimir V Gureev1, Vladimir M Pokrovskii1, Evgenii A Patrachanov1, Igor B Kovalenko1, Vladimir I Shutov1 and Alexey A Shabalin2
1Department of Medical and Dental Science, Belgorod State National Research University, Belgorod, Russia2Department of Medical and Dental Science, Kursk State Medical University, Marx St, Kursk, Russia
Citation: Tatiana G Pokrovskaya*, Taisiya A Khadieva, Vladimir V Gureev, Vladimir M Pokrovskii, Evgenii A Patrachanov, Igor B Kovalenko, Vladimir I Shutov, Alexey A. Shabalin, Correction of the Endothelial Function and the Hemostasis System Disorders with Ademethionin and Taurine in Model Associated with ADMA-Like Preeclampsia, J Res Med Dent Sci, 2021, 9(11): 175-181.
Received: 01-Dec-2021 Accepted: 15-Dec-2021 Published: 22-Dec-2021
