Research Article - (2022) Volume 10, Issue 7
Comparison of the Bactericidal Effects of Two Different Diode Laser Wavelengths 810 NM And 980 NM within the Treatment of E. Faecalis-Infected Root Canals
Murtadha Mustafa Thamer* and Salah Alkurtas A
*Correspondence: Murtadha Mustafa Thamer, Department of Biomedical Applications, Institute of Laser for Postgraduate Studies, University of Ba, Iraq, Email:
Abstract
Background: A variety of causes can lead to endodontic treatment failure, including bacterial persistence, insufficient root canal cleansing or obturation, incorrect coronal seal, and untreated canals (missed canals). The endodontic treatment failure arises from many reasons and the main one is the existence of some bacterial species inside the root canals like the Enterococcus faecalis , which is the most common type. It possesses unique characteristics that enable it to evade disinfection leading to radicular inflammation. Newer laser technology disinfection techniques have been recommended to be effective for routine endodontic treatment as a result of the development of the potent antimicrobial capabilities of lasers in recent years.
Aim: In vitro study, comparative between the influences of two various wavelengths of diode laser (810 nm and 980 nm) within the root canal system against Enterococcus faecalis during endodontic treatment.
Materials and methods: Preparation and sterilization of a total of forty canals before being contaminated with Enterococcus faecalis bacteria and cultured for two weeks. After that, the human permanent teeth were separated into four groups at random. A (control group) specimens that have not been treated, group B (its specimens were treated with 17% EDTA and sodium hypochlorite at 5.25%), group C (specimens radiated with 810 nm diode laser), and Group D (specimen radiated with 980 nm diode laser). After the disinfection steps, specimens were plated on blood agar media in order to count the number of colonies for experimental groups.
Results: In both experimental groups, laser irradiation reduced the number of bacterial colonies. The reduction in the microbial count was significantly greater in the 810 nm laser group (70.8%) compared to the 980 nm laser group (29.1%). Using Dunnett's T3 test, which demonstrated significant differences among the groups with an exception of that between-group C and other groups which was not a significant difference. However, the greatest bacterial eradication was achieved when sodium hypochlorite was used in conjunction with the EDTA treatment (81.6% CFU/ml reduction).
Conclusion: The 810 nm diode lasers sterilized and killed E. Faecalis bacteria more successfully than the 980 nm diode laser. However, when sodium hypochlorite at 5.25% was utilized in combination with the 17% EDTA treatment, the most bacterial clearance was observed.
Keywords
α-SMA, CO2, Laser diode, Laser, Mucosal epitheliumIntroduction
Because of its complex anatomy, bacteria's ability to penetrate dentinal tubules and a smear layer that forms through instrumentation of root canal by endodontic files, disinfecting the root canal system is difficult. The basic purpose of root canal treatment is to remove pathogens and necrotic pulp tissue remnants from the root canal system. However, it has been established that 35% of the root canal surface area stays unchanged after endodontic preparation with a rotary Ni-Ti system. The performance of perfect bacterial eradication inside the root canal system is limited by endodontic methods, systems, and disinfection solutions used during root canal therapy. Various types of endodontic irrigants like Normal saline, Sodium hypochlorite, chlorhexidine, citric acid, and EDTA are employed in chemical disinfection protocols. These chemicals may be useful in specific state but ineffective in another [1].
As a result, one of them cannot rely on oneself completely. There is a wide difference between the capacity of pathogens to penetrate dentinal tubules and the ability of chemical irrigants to infiltrate and sterilize within these tubules, which is less owing to surface tension. As a result, it is regarded ineffective against these bacteria. The possibility of irrigants passing from the apical foramen to the periarticular area must be considered, making the apical disinfection process more complicated 6. The irrigant solution diffuses slowly through dentinal tissue, and it is influenced by several factors such as irrigant concentration and temperature.
Certain bacterial species have the ability to resist surrounding conditions such as nutritional starvation and alkaline pH through special mechanisms such as the active protein pump found in the cellular membrane of Enterococcus faecalis , This is thought to explain why, in addition to their capacity to penetrate the dentin and their high resistance to chemical solutions and medications like calcium hydroxide, these bacteria are among the most predominant kinds founded in re-infected root canals of endodontically treated teeth [2].
New methods have been discovered in the root canal sterilization process, such as laser, to obtain better results than the results of chemical sterilization. For dental application the diode laser range from 800 nm to 1064 nm, which is the best type compared to other types of laser because of its small size, flexible fibres, and wide output power range, High penetration into dentinal tubules, adequate antibacterial action, and Large-scale E. faecalis eradication. A number of studies have proven the effective thermal properties of diode laser against bacteria, which were reported that the bacteria lodged to a depth of 500 m in dentinal tubules were successfully eliminated by diode laser either alone or in conjunction with other chemical irrigants [3].
Also, 980 nm diode lasers is well absorbed by water but is slightly absorbed by hydroxyapatite crystals; this results in light scattering in dentin [4].
Aim of the study
A comparison of the bactericidal effects of two different diode laser wavelengths within the treatment of E. faecalis- infected root canals, in vitro study [5].
Materials and Methods
Preparation step
The identical size and completely developed apices of 40 anterior or premolars removed single canal permanent teeth that weren't treated endodontically were utilized during this research. All teeth were debrided for a half-hour in 5.25% NaOCl (Chloraxid 5.25%, Cerkamed, Cerkamed, and Poland). To encourage a similar working length for all tooth specimens, the working lengths were established to a 15 mm length, and thus the specimens were interrupted at the cement enamel junction with a disc bur employing a high-speed hand piece. Then, the teeth were immersed in normal saline (0.9% sodium chloride) at ambient temperature till the subsequent step. A 15 K-file was used for working length determination. The K-file was inserted through the tooth canal until its end could be seen at the apical foramen of the root [6].
Protaper universal rotary file system was used to prepare the teeth canals. After each file, 3 ml of 5.25% of NaOCL was injected employing a 27-gauge irrigation needle (Lingchen, China). To ensure that each canal got the same total irrigation duration, the irrigation needle was positioned 1 mm from the apical foramen and delivered at a rate of three ml/min. Then, for 4 minutes, 2 ml of 17% EDTA was injected into each canal, followed by final irrigation with 5.25% NaOCl with an ultrasonic tip; both solutions (EDTA and NaOCl) were activated for 30 seconds. Finally, all specimen canals were washed with sterile water and dried with sterile paper points [7].
Composite resin fillings (Z350, 3 M, and USA) were used to close the apical foramens. After the cleaning and shaping step and each one among the specimens was autoclaved for 20 minutes at 121°C under 15 psi pressures. After sterilization, each specimen was placed in an Eppendorf tube with 2 mL of sterile Luria-Bertani broth (LB broth) and incubated at 37°C for 48 hours with daily checks to form sure that the broth is free of turbidity [8].
Experimental contamination and incubation step
A 15 K-file was used to isolate the Enterococcus faecalis from infected root canals with a circumferential filing motion for 20 seconds and inoculating each sample with 20 L of LB broth. After inoculating the suspension in Pfizer Selective Enterococcus media (PSE agar), the E. faecalis bacteria were identified using Vitek. Spectrophotometric dilution of the bacterial suspension to match 0.5 McFarland standard turbidity (1.5 × 108 CFU/mL). After injecting this bacterial suspension into the canals of prepared teeth, the orifices were temporarily filled with light-cured fillings after being dry [9].
The specimens placed into the Eppendorf tube contain 2 ml of LB broth and were incubated at 37°C under anaerobic conditions for 14 days. The broth and the tube were changed every three days for nutrition purposes [10].
Selection of Laser parameters and pilot study
Diode lasers have the most frequent and effective powers of 1, 1.5, and 2 watts Continuous Emission (CW), with exposure durations ranging from 5 to 10 seconds, Because laser radiation raises the temperature of the external root surface, a pilot study was done to see how much these powers could raise the temperature on the external root surface and to choose the appropriate power to ensure that our power was still safe for periodontal tissues and within the biological limit. A single canal permanent tooth was cleaned and prepared in the same way as the other teeth specimens in this study, then placed in a stone mold and connected to a thermocouple wire at the root's thinnest surface in this pilot study (the mesial root surface). A thermocouple wire is attached to a digital thermometer that is highly accurate (prosKit, MT-2010, USA). The diode Laser fibre (size 200 m) was inserted into the root canal until the apical foramen (one millimetre above the apex) and for four times, the laser beam was delivered in a helicoid motion down and upward for five seconds of exposure time, followed by a 20-second resting interval [11-15].
The following are the results of powers that were tested with the maximum temperature elevation gained by each power, Tables 1 and 2.
| Powers | Maximum |
|---|---|
| temperature elevation | |
| 0.5 watt | 3.5°C |
| 1 watt | 6.8°C |
| 1.5 watt | 23°C |
Table 1: 810 nm diode lasers.
| Powers | Maximum temperature elevation |
|---|---|
| 0.5 watt | 2.5°C |
| 1 watt | 5°C |
| 1.5 watt | 19.8°C |
Table 2: 810 nm diode lasers.
Tables 1 and 2 show the maximum temperature elevation of the external root surface above the room temperature during laser irradiation. Previous research has shown that a temperature increase of 10°C above body temperature for 1 minute causes irreversible damage to the alveolar bone. The results showed that when using a water bath and a laser power of either (1.5) W CW or (2.5) W/20 Hz, the maximum temperature did not exceed 8.5°C with a radiation duration of only 20 seconds and that the used powers would be very safe if we added the body effect of cooling by circulation and heat dissociation. We used dry temperature measurement experiments with an ambient room temperature of (21°C ± 2°C) in this study. According to other studies, the maximum temperature increase tolerated by periodontal tissues should be less than 7 degrees. However, the appropriate power was 1 watt to ensure that the temperature did not exceed the periodontal tissues' maximum temperature elevation tolerance and did not rise more than 10 degrees Celsius above body temperature [16].
Sterilization of experimental samples
The tooth specimens were removed from the incubator after two weeks of anaerobic incubation and immersed in CHX solution for two minutes before being cleaned with sterile water and split into four groups, each having ten teeth specimens. Group A (control group) specimens that have not been treated, group B (its specimens were treated with sodium hypochlorite at 5.25% and 17% EDTA), group C (specimens radiated with 810 nm diode laser), and group D (specimen radiated with 980 nm diode laser).
Group A, control group (n=10)
To compare the bacterial reduction rate of the other three experimental groups, assume that the infection rate of teeth specimens with E. faecalis is 100%.
Group B (n=10): NaOCL and EDTA
For canal disinfection, this group used 2 ml of 17% EDTA for 3 minutes and 3 ml of 5.25% sodium hypochlorite for another 3 minutes respectively using a 30-gauge irrigation needle with a lateral opening at the closed end of it then irrigates with 3 ml of 0.9% normal saline. After drying the canals with sterile paper points, 0.1 ml of sterile water (0.9% normal saline) was injected into each canal, which was then sealed with a temporary filling and incubated under anaerobic conditions for 24 hours [17].
Group C (n=10): 810 nm diode laser
The laser group's tooth specimens were disinfected using an 810 nm diode laser with a 200 μm endodontic fibre tip and a 1 watt output power in Continuous Emission Mode (CW). Following the removal of temporary fillings, each canal was filled with 0.1 ml of sterile water and irradiated four times with a 5 seconds laser exposure, followed by a 20 seconds interval between each exposure. The laser tip was inserted 1 millimetre below the working length into the root canal and moved downward and upward in a helicoid pattern [18]. Following the lasing procedure, each specimen was received 0.1 ml of sterile water, then sealed with temporary filling restoration and incubated under anaerobic conditions for 24 hours.
Group D (n=10): 980 nm diode laser
The specimens irradiated with a 980 nm diode laser with an endodontic fibre tip (200 μm) with an output power of 1 watt at Continuous Emission Mode (CW) with 5 seconds exposure time four times followed by 20 seconds resting interval for each exposure. The procedure was the same in group C [19].
Determination of bacterial count
After a 24 hours incubation period, all specimen groups were brought out and their temporary fillings were removed, and 0.1 mL of sterile normal saline was used as a transport medium inside the canals. To disrupt the bacterial biofilm and collect the dentin chips, 25 K-File was inserted inside each canal and filed circumferentially for 30 seconds. A F3 sterile paper point was used for each canal to collect the dentin chips with their transporting media. After that, each canal's paper point and k-file were inserted into an Eppendorf tube containing 2 ml of sterile LB broth. The Eppendorf tube was shaken for 1 minute in a vortex mixer before being incubated under anaerobic conditions for 24 hours [20].
After incubation, 0.5 ml of each Eppendorf tube was taken (using a micropipette) and tenfold serially diluted before being inoculated in a plate containing blood agar and incubated for 24 hours. The number of Colony-Forming Units (CFU) was calculated after the incubation period using the equation [21].
{Number of colonies present on the plate X Dilution factor=No. of CFU/ml}
We compared the means of groups B, C, and D to the mean of group A to calculate the reduction in bacterial colonies in each disinfected experimental group (Control group).
Statistical analysis
To match the mean CFUs/ml among the groups, the researchers used a One-Way Analysis Of Variance (ANOVA) model. Dunnett's T3 post hoc test was used to make multiple comparisons of mean CFU/ml between groups. A statistically significant P value of less than 0.05 was used. SPSS for Windows version 21.0.0 was used to perform the statistical analysis [22-26].
Results
Evaluation of bacteriological growth after disinfection
In comparison to the other disinfected groups, group D (17% EDTA and 5.25% NaOCL) had less bacterial growth on the blood agar media after 24 hours of incubation. Table 3 shows the number of CFUs that were counted after collecting bacterial colonies on the blood agar plates after a tenfold serial dilution [27].
| AControl group | BEDTA+NaOCL | C810 nm group | D980 nm group |
|---|---|---|---|
| 23200 | 4000 | 6600 | 20600 |
| 20900 | 3400 | 7900 | 16800 |
| 18000 | 5700 | 4100 | 11500 |
| 26500 | 5200 | 7000 | 18300 |
| 16300 | 4800 | 4300 | 15000 |
| 13200 | 3200 | 8200 | 10800 |
| 28700 | 2800 | 6400 | 16800 |
| 14800 | 2400 | 4500 | 9400 |
| 15100 | 2500 | 4200 | 10200 |
| 22000 | 3600 | 3700 | 15700 |
Table 3: number of CFUs/ml in all specimens.
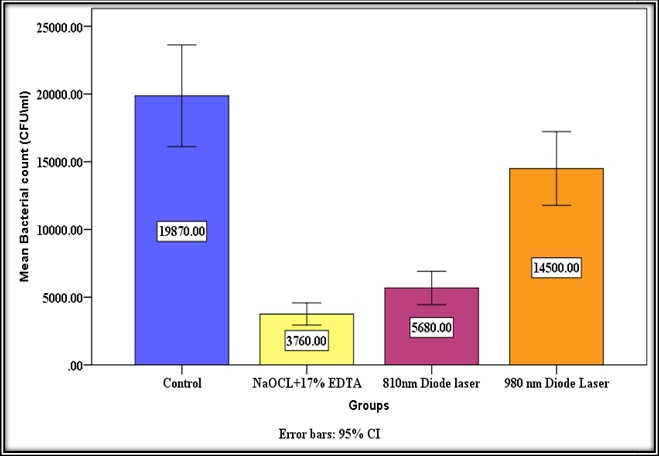
Table 4 shows descriptive statistics for CFUs/ml, such as mean, Standard Deviation (SD), Standard Error (SE), minimum values, and maximum values (Figure 1) [28].
| Groups | N | Mean | ± SD | ± SE | Minimum | Maximum |
|---|---|---|---|---|---|---|
| Control | 10 | 19870 | 5247.232 | 1659.32 | 13200 | 28700 |
| NaOCL+17% EDTA | 10 | 3760 | 1145.232 | 362.154 | 2400 | 5700 |
| 810 nm Diode laser | 10 | 5680 | 1719.044 | 543.609 | 3700 | 8200 |
| 980 nm Diode Laser | 10 | 14500 | 3810.22 | 1204.897 | 9400 | 20600 |
Table 4: Descriptive statistics of bacterial count among groups.
Figure 1: Mean bacterial count.
To measure the bacterial decrease in each treated group, comparisons between the mean of a control group and the mean of treated groups may be done using the Table and Figure above. Group D had the highest bacterial decrease, with 81.6% of bacteria destroyed, followed by groups B (70.8% bacterial reduction) and C (29.1% bacterial reduction), in that order. It's clear that combining NaOCl and EDTA inside infected root canals has a powerful bactericidal effect against E. faecalis biofilm. Using One Way Analysis Of Variance (ANOVA), a statistical test of CFU across groups indicated a very significant difference of p=0.00. Dunnett's T3 post hoc test was used to do multiple comparisons of CFU/ml across groups (Table 5). Except for the comparison between the control group and the 980 nm laser group, which was not significant (p=0.098), other comparisons with the control group indicated highly significant [29-35].
| Multiple comparisons of Bact. Between groups using Dunnett T3 post hoc test. | |||
|---|---|---|---|
| Groups | Mean Difference (I-J) | p value | |
| Control | 810 nm Diode laser | 14190 | 0.000* |
| 980 nm Diode Laser | 5370 | 0.098^ | |
| NaOCL+17% EDTA | 16110 | 0.000* | |
| NaOCL+17% EDTA | 810 nm Diode laser | -1920 | 0.054^ |
| 980 nm Diode Laser | -10740 | 0.000* | |
| 810 nm Diode laser | 980 nm Diode Laser | -8820 | 0.000* |
| *=significant at p<0.05, ^=not significant at p>0.05 | |||
Table 5: The Dunnett T3 post hoc test was used to compare CFU between groups.
Discussion
Despite the fact that a variety of chemical agents with various properties are available, none of the currently available irrigating solutions can be considered optimal, or even close to it, when it comes to clean the root canal. In order to contribute as much as possible to the success of root canal treatment in clinical practice, a combination of solutions must be used in a specific order. E. faecalis survive mechanical and chemical root canal preparation because of its ability to penetrate deep into dentinal tubules and form a biofilm. However, after one year of therapy, 20-23% of patients with endodontic failure re-infected root canal had E. faecalis inside their treated root canals. The smear layer is a film of material adhered to the dentin surface after root canal instrumentation, consisting of discarded dentin fragments [36]. Remains of live or necrotic pulp tissue, bacteria (including E. feacalis ) and their metabolites, and chemical irrigants. The smear layer functions as a barrier between the root filling and the canal wall, preventing root canal irrigants from accessing the root filling and acting as a potential path of bacterial contamination between the two surfaces. The difficulty of eliminating the smear layer inside the apical area may be attributed to the inability to administer agents such as NaOCl and EDTA due to the reduced dimensions of the apical canal, which obstructs irrigation delivery [37-43]. Due to multiple benefits such as smear layer removal, bacterial count reduction, and apical micro leakage reduction, laser therapy is widely used in endodontic treatment. Because of their small size and low cost, diode lasers are very popular. They also have a flexible and thin fibre that allows easy access to narrow canals and improves disinfection efficacy in the radicular dentinal tubules to a depth of 500 µ [44-46].
Diode laser is recommended for endodontic treatment because its wavelength is in the infrared range and its thin and flexible fibres help removes the smear layer. In this in vitro study, results showed that using diode lasers with wavelengths of 810 nm and 980 nm at a power of 1 Watt reduced E. faecalis bacterial colonies in the root canal system when compared to a control group. The effect of a diode laser at 810 nm on reducing E. faecalis colony counts was significantly greater than that of a diode laser at 980 nm. The reduction of the bacterial count was 70.8% CFU with 810 nm diode laser while the bacterial reduction was 29.1% CFU with 980 nm diode laser under the same conditions [47].
The effects of diode lasers with wavelengths of 810 nm and 980 nm on intra canal E. faecalis have been studied in various studies. In 2018 by using a diode laser, they were able to reduce the bacterial count in deep layers of an infected root canal wall by 74% (810 nm). Under the same conditions, despite using a higher distal output power, the 980 nm laser reduced the bacterial count by 57%. Diode lasers have a low absorption coefficient in water (a=0.04-0.05 cml), which means they have low absorption in dentin. The superior bactericidal effect of diode laser irradiation (more than 1000 m into dentinal tubules) may be due to its greater depth of penetration [48].
These lasers can directly interact with root canal pathogen pigments (e.g. melanin) and have a strong bactericidal effect. They also cause thermal photo disruption within the unreachable parts of tooth canal dentin, leading to an enhanced bactericidal effect 40. Another study compared the antibacterial effects of intra canal irrigants and diode lasers on infected root canals in 2014. They used a diode laser with an output power of 1.5 W and a frequency of 20 Hz that had an output wavelength of 830 nm. They found that while diode lasers were not as effective as irrigants in disinfecting root canals, they did show increased disinfection in deep dentin due to deeper penetrating depth. Sodium Hypochlorite (NaOCL) is still the most prevalent root canal irrigant. Sodium hypochlorite is a popular endodontic irrigating solution because of its ability to digest organic tissues during chemo-mechanical root canal debridement. The optimal chemical concentration of NaOCl is between 1% and 6%.
Studies have shown that a concentration of 5.25% NaOCl can kill E. Faecalis and C. Albicans within 15-30 seconds. The synthetic amino acid Ethylene Diamine Tetra Acetic Acid (EDTA) is frequently used as a chelating agent. By chelating calcium ions, EDTA demineralizes the inorganic components of dentin, lowering the micro hardness. Within one minute, the EDTA solution can completely remove inorganic components from the smear layer and open dentinal tubules. However, a lengthy treatment (more than 10 minutes) may cause inter tubular and peritubular dentin erosion. Sodium hypochlorite remains the most important chemical irrigant. Because iodine and chlorhexidine do not dissolve organic tissue, they are believed to be softer on soft tissues than sodium hypochlorite. Antimicrobial irrigants such as NaOCl are not replaced by chelators in liquid form. Chelators have low antimicrobial properties, but they can be used to remove the smear layer, allowing other irrigants, such as NaOCl, to penetrate deeper and thus increase their antimicrobial effect [49].
EDTA concentrations of 15-17% can eliminate the inorganic component of the smear layer, whereas NaOCl concentrations surpassing 1 percent can remove the organic portion. This study utilized 2 ml of 17% EDTA and 3 ml of 5.25% sodium hypochlorite for root canal disinfection. The reduction in bacterial counts was 81.6% CFU. In a similar study, in root canal disinfection and E. faecalis eradication, diode laser irradiation along with chemo mechanical irrigation was found to be more efficient than NaOCl irrigation alone. In vivo study in 2018 to assess the diode laser's antibacterial effect on the infected root canal wall. In this experiment, a diode laser was used. The findings revealed that a combination of NaOCl irrigation and laser irradiation is more effective than traditional endodontic therapy in reducing bacterial flora in the root canal system [50].
Conclusion
The bactericidal effect of 810 nm was greater than 980 nm when comparing the effects of diode laser radiation on E. faecalis in the root canal system at 1 watt. Under the same conditions, 810 nm diode lasers reduced bacterial count by 70.8% CFU, while 980 nm diode lasers reduced bacterial count by 29.1% CFU. However, 5.25% sodium hypochlorite with 17% EDTA has a better antibacterial effect on E. faecalis biofilm (The reduction of the bacterial count was 81.6%) than diode lasers. When diode lasers were used in conjunction with other chemical irrigants like NaOCL and EDTA, the results showed that they had a greater effect on E. faecalis reduction.
References
- Torabinejad M, Handysides R, Khademi AA, et al. Clinical implications of the smear layer in endodontics: A review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002; 94:658-666.
- Vahdaty A, Ford TP, Wilson RF, et al. Efficacy of chlorhexidine in disinfecting dentinal tubules in vitro . Dent Traumatol 1993; 9:243-248.
- Cumbo E, Cusimano P, Russo R, et al. Effects of two Ni-Ti preparation techniques on root canal geometry assessed by electronically profiling the endodontic space. Int Endod J 2006; 34:221-230.
- PETERS Ove A, Schonenberger K, Laib A, et al. Effects of four Niâ??Ti preparation techniques on root canal geometry assessed by micro computed tomography. Int Endod J 2001; 34.3:221-230.
- Ordinola Zapata R, Bramante CM, Brandao Garcia R, et al. The antimicrobial effect of new and conventional endodontic irrigants on intra-orally infected dentin. Acta Odontol Scand 2013; 71:424-431.
- Elio B, Riccardo M, Alessandra A, et al. Penetration ability of different irrigants into dentinal tubules. J Endod 1997; 23:725-727.
- Haapasalo M. Irrigation in endodontics. Br Dent J 2014; 216:299-303.
- Van Der Sluis LW. Endodontics in motion: New concepts, materials and techniques 3. The role of irrigants during root canal treatment. Ned Tijdschr Tandheelkd 2015; 122:533-538.
- Evans M, Davies JK, Sundqvist G, et al. Mechanisms involved in the resistance of Enterococcus faecalis to calcium hydroxide. Int Endod J 2002; 35:221-228.
- Flanagan D. Enterococcus faecalis and dental implants. J Oral Implantol 2017; 43:8-11.
- Ran S, Gu S, Wang J, et al. Dentin tubule invasion by Enterococcus faecalis under stress conditions ex vivo . Eur J Oral Sci 2015; 123.5:362-368.
- Weckwerth PH, Zapata RO, Vivan RR, et al. In vitro alkaline pH resistance of Enterococcus faecalis . Braz Dent J 2013; 24:474-476.
- Preethee T, Kandaswamy D, Arathi G, et al. Bactericidal effect of the 908 nm diode laser on Enterococcus faecalis in infected root canals. J Conserv Dent 2012; 15:46.
- Clinical implications of the smear layer in endodontics: A review
- Beer F, Buchmair A, Wernisch J, et al. Comparison of two diode lasers on bactericidity in root canals-an in vitro study. Lasers med sci 2012; 27:361-364.
- Schoop U, Kluger W, Dervisbegovic S, et al. Innovative wavelengths in endodontic treatment. Lasers in Surgery and Medicine. J Amer Soci Laser Med Surg 2006; 38:624-630.
- Asnaashari M, Safavi N. Disinfection of contaminated canals by different laser wavelengths, while performing root canal therapy. J Lasers Med Sci 2013; 4.1:8.
- Gutknecht N, Van Gogswaardt DI, Conrads G, et al. Diode laser radiation and its bactericidal effect in root canal wall dentin. J Clin Laser Med Surg 2000; 18:57-60.
- Haapasalo M, Shen Y, Wang Z, et al. Irrigation in endodontics. Br Dent J 2014; 216:299-303.
- Van Der Sluis LW. Endodontics in motion: New concepts, materials and techniques 3. The role of irrigants during root canal treatment. Ned Tijdschr Tandheelkd 2015; 122:533-538.
- Gutknecht N, Franzen R, Meister J, et al. Temperature evolution on human teeth root surface after diode laser assisted endodontic treatment. Lasers med sci 2005; 20:99-103.
- Sheimaâ??a A, Al Maliky MA, Mahmood AS, et al. Temperature elevation investigations on the external root surface during irradiation with 940 nm diode laser in root canal treatment. Saudi Endod J 2018; 8:14.
- Gutknecht N, Franzen R, Meister J, et al. Temperature evolution on human teeth root surface after diode laser assisted endodontic treatment. Lasers med sci 2005; 20:99-103.
- Krishnan SN, Nayarisseri A, Rajamanickam U, et al. Biodegradation effects of o-cresol by Pseudomonas monteilii SHIES on mustard seed germination. Bioinfo 2018; 14:271.
- Karaman R, Khamis M, Abbadi J, et al. Paracetamol biodegradation by activated sludge and photocatalysis and its removal by a micelle-clay complex, activated charcoal, and reverse osmosis membranes. Environ Technol 2016; 37:2414-2427.
- Haapasalo M. Irrigation in endodontics. Br Dent J 2014; 216:299-303.
- Figdor D, Davies JK, Sundqvist G, et al. Starvation survival, growth and recovery of Enterococcus faecalis in human serum. Oral Microbiol Immun 2003; 18:234-239.
- Ng YL, Mann V, Rahbaran S, et al. Outcome of primary root canal treatment: Systematic review of the literature-Part 2. Influence of clinical factors. Int Endod J 2008; 41:6-31.
- Kokkas AB, Boutsioukis AC, Vassiliadis LP, Stavrianos CK. The influence of the smear layer on dentinal tubule penetration depth by three different root canal sealers: an in vitro study. J endod 2004; 30:100-102.
- Wang Z, Shen Y, Haapasalo M, et al. Effect of smear layer against disinfection protocols on Enterococcus faecalisâ?? infected dentin. J endod 2013; 39:1395-1400.
- Lui JN, Kuah HG, Chen NN, et al. Effect of EDTA with and without surfactants or ultrasonics on removal of smear layer. J endod 2007; 33:472-475.
- Yuichi Kimura. Treatment of dentine hypersensitivity by lasers: a review. J Clin Periodontol 2000; 27:715-721.
- Muhammad OH, Rocca JP, Fornaini C, et al. Evolution of the role of phototherapy during endodontic decontamination. Laser Ther 2015; 24:291-302.
- De Souza EB, Cai S, Simionato MR, et al. High power diode laser in the disinfection in depth of the root canal dentin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008; 106:68-72.
- Wang X, Sun Y, Kimura Y, et al. Effects of diode laser irradiation on smear layer removal from root canal walls and apical leakage after obturation. Photomed Laser Surg 2005; 23:575-581.
- Martins MR, Franzen R, Depraet F, et al. Rationale for using a double-wavelength (940 nm+2780 nm) laser in endodontics: literature overview and proof-of-concept. Lasers Dent Sci 2018; 2:29-41.
- Gutknecht N, Franzen R, Schippers M, et al. Bactericidal effect of a 980-nm diode laser in the root canal wall dentin of bovine teeth. J Clin Laser Med Surg 2004; 22:9-13.
- Gutknecht N, Van Gogswaardt DI, Conrads G, et al. Diode laser radiation and its bactericidal effect in root canal wall dentin. J Clin Laser Med Surg 2000; 18:57-60.
- Bago Juric I, Ivica Anic. The use of lasers in disinfection and cleaning of root canals: a review. Acta Stomatol Croat 2014; 48:6-15.
- Mehrvarzfar P, Saghiri MA, Asatourian A, et al. Additive effect of a diode laser on the antibacterial activity of 2.5% NaOCl, 2% CHX and MTAD against Enterococcus faecalis contaminating root canals: an in vitro study. J Oral Sci 2011; 53:355-360.
- Lopez Jimenez L, Arnabat DomÃnguez J, Viñas M, et al. Atomic force microscopy visualization of injuries in Enterococcus faecalis surface caused by Er, Cr: YSGG and diode lasers. Med Oral Patol Oral Cir 2015; 20:45.
- Ashofteh K, Sohrabi K, Iranparvar K, et al. In vitro comparison of the antibacterial effect of three intracanal irrigants and diode laser on root canals infected with Enterococcus faecalis . Iran J Microbiol 2014; 6:26.
- Rahimi S, Janani M, Lotfi M, et al. A review of antibacterial agents in endodontic treatment. Iran Endod J 2014; 9:161.
- Podar R, Kulkarni GP, Dadu SS, et al. In vivo antimicrobial efficacy of 6% Morinda citrifolia, Azadirachta indica, and 3% sodium hypochlorite as root canal irrigants. Eur J Dent 2015; 9:529-534.
- Hulsmann M, Heckendorff M, Lennon A, et al. Chelating agents in root canal treatment: Mode of action and indications for their use. Int Endod J 2003; 36:810-830.
- Cruz Filho AM, Sousa Neto MD, Savioli RN, et al. Effect of chelating solutions on the microhardness of root canal lumen dentin. J endod 2011; 37:358-362.
- Adarsh V, Kiran MM, Jamsheed ET, et al. A Comparative evaluation of smear layer removal by using three different irrigating systems in endodontics: An in-vitro scanning electron microscopic study. J Int Oral Health 2016; 8:80.
- Bergmans L, Moisiadis P, Teughels W, et al. Bactericidal effect of Nd: YAG laser irradiation on some endodontic pathogens ex vivo. Int Endod J 2006; 39:547-557.
- De Souza EB, Cai S, Simionato MR, et al. High power diode laser in the disinfection in depth of the root canal dentin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008; 106:68-72.
- Tilakchand M, Singh NN, Yeli MM, et al. Evaluation of the antibacterial efficacy of EZLASE diode LASER on the infected root canal system: An in vivo study. J Conserv Dent 2018; 21:306.
Author Info
Murtadha Mustafa Thamer* and Salah Alkurtas A
Department of Biomedical Applications, Institute of Laser for Postgraduate Studies, University of Ba, IraqCitation: Murtadha Mustafa Thamer, Salah Alkurtas A, Comparison of the Bactericidal Effects of Two Different Diode Laser Wavelengths 810 NM And 980 NM within the Treatment of E. Faecalis -Infected Root Canals, J Res Med Dent Sci, 2022, 10 (7): 007-012.
Received: 23-Apr-2022, Manuscript No. JRMDS-22-43719; , Pre QC No. JRMDS-22-43719; Editor assigned: 25-Apr-2022, Pre QC No. JRMDS-22-43719; Reviewed: 09-May-2022, QC No. JRMDS-22-43719; Revised: 23-Jun-2022, Manuscript No. JRMDS-22-43719; Published: 01-Jul-2022