Research Article - (2022) Volume 10, Issue 9
Comparative Evaluation of Remineralization of White Spot Lesion in Nano Silver Fluoride and Nano Hydroxyapatite Varnish: An In vitro Study
*Correspondence: Rama Raji Sankaranarayanan, Department of Implantology, Rajiv Gandhi University of Health Sciences, Chennai, Tamil Nadu, India, Email:
Abstract
Introduction: White spot lesions are a common finding with orthodontic treatment. Proper maintenance of oral hygiene, regular scaling, usage of fluoridated toothpaste, application of varnish etc. have been used to prevent, reduce or re-mineralize white spot lesions. Unfortunately, a method to completely prevent white spot lesions has yet to be achieved.
Aim: To determine and compare the efficacy of re-mineralization of white spot lesions between two green synthesized nanoparticle varnishes namely nano silver fluoride and nano hydroxyapatite varnish.
Materials and methods: 30 samples of extracted human premolar teeth were subjected to demineralization solution for 72 hours to artificially induce an incipient lesion. They were randomly divided into 3 groups 1) Green synthesized NaF; 2) Green synthesized HAP; 3) Control (no treatment). A total of 9 random samples were selected and subjected to Vickers microhardness test and an atomic force microscope to analyse the surface micro-hardness test and analysed with one way ANOVA and repeated measures ANOVA. Level of significance was set at p=0.05.
Results: At T1, the surface micro-hardness test values of all samples was not statistically different among the groups (p=-0.8). Mean of surface micro-hardness of induced white spot lesion was 125.7 ± 16.5 were significant than baseline surface micro-hardness test values (p<0.001). The highest surface micro-hardness test values were observed in green synthesized sodium silver fluoride group (mean 192.6 ± 29.86). The surface micro-hardness of green synthesized HAP was 171.5 ± 20.66 and that of the control group was 113.2 ± 19.3. Intergroup analysis shows no significant difference between green synthesized sodium silver fluoride and green synthesized hydroxyapatite (p=0.001).
Conclusion: Green synthesized sodium silver fluoride n-NSF can be considered as an option for arresting white spot lesions in orthodontic patients. However, more in-vivo studies are needed to review the long term effects of the varnish.
Keywords: Nanoparticle varnish, Caries prevention, Sodium fluoride, Hydroxyapatite, White tea
Introduction
White spot lesion is defined as “white opacity”, occurring as a result of a subsurface enamel demineralization that is located on the smooth surface of teeth [1]. The white opacity is the change in the light scattering optical properties due to demineralization of the enamel [2]. Fermentable carbohydrates, acid producing bacteria, poor oral hygiene, low pH of the saliva and diet high in sugar contribute to such incipient lesions [3]. Many research shows that white spot lesions develop due to long standing undisturbed plaque accumulation [4,5]. This creates an environment which leads to decrease in pH, where the acid penetrates underneath the plaque causing demineralization of the enamel followed by cavitation which can occur in 4 weeks [6,7].
Studies have explained the pathophysiology of caries where there was presence of bacteria in an acid environment in the plaque or biofilm [8,9]. Orthodontic brackets are one of the most important factors of food and debris lodgement which may tend to aggravate the white spot lesion. It becomes a high priority to maintain oral hygiene [10-12]. After the removal of these brackets and bands there is remineralization of the lesion which becomes less opaque but not completely. Hence, it becomes really important for us to arrest such lesions in the beginning [14].
Fluoride varnishes have been widely used to arrest enamel lesions with formation of Fluor apatite [15]. Varnish is preferred over other methods due to increased contact between teeth and varnish [16]. This forms a protective covering over the teeth thus increasing the prophylactic effect of the varnish. Nanotechnology is the new emerging field of science where the particles are microsized measured in nanometres. Nanoparticle was first introduced by Nobel laureate Richard P. Feyman in his famous lecture in 1959: “There’s plenty of room at the bottom” [17]. Nanoparticles are substances which are less than 100 nm. These are available in 0D, 1D, 2D, 3D depending on the shapes [18]. In recent times, nanoparticles have been incorporated in dental varnish which increases the ability to penetrate deep into the enamel surface rather than just coating superficially. Considering the toxicity of synthetic particles nanoparticles have been created from naturally occurring Camelia sinesis (White tea) incorporated with sodium silver and hydroxyapatite particles [19].
Materials and Methods
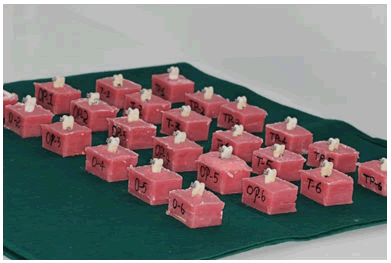
This in-vitro study was approved by the university ethical committee. The study used sound extracted human premolar teeth from patients who underwent orthodontic treatment. Sample size was N=30 which was calculated using G power software, post hoc test. Teeth with enamel defects and decay were excluded from the study [20]. The specimens were washed and disinfected in 0.1% thymol. They were then mounted on epoxy resin such that the buccal surface was exposed (Figure 1). Later, tooth mounted resin models were polished with silicon carbide bur (2400 grit) and washed in distilled water for 20 seconds.
Figure 1: Specimens mounted on epoxy resin.
The baseline surface micro-hardness test (T1) was done using Vickers microhardness tester (Mitutoyo HM100, Japan) (Figure 2) under 50 grams load for 10 seconds. Surface microhardness test was analysed at 4 different random points on the crown surface. The first indentation was at the centre of the enamel and the other points anywhere around at a distance of 300 um. Surface Microhardness test (SMH) was performed by a single operator and samples were selected whose SMH was in the range of 262 to 389 according to ten cates. Samples which had SMH below this were excluded from the study [21].
Figure 2: Specimens loaded for Vickers microhardness test 50 grams for 10 seconds.
Production of artificial carious lesion (T2)
The demineralizing solution (Figure 3) was prepared with 50 mM acetic acid, 2.2 mM potassium dihydrogen phosphate, 2.2 mM calcium nitrate and 0.1 ppm NaF and the specimens were immersed in the solution for 3 hours. After this the specimens were immersed in a remineralization solution containing CPP-ACP for 21 hours. It was allowed to air dry after which Ormco brackets were bonded to the sample teeth using an orthodontic adhesive. This was followed with a surface microhardness test (T2).
Figure 3: Preparation of demineralizing solution.
The 30 samples were divided into 3 groups with 10 in each group and were subjected to their respective varnish application protocols as follows [22].
Group 1: Control group (where no varnish application was done).
Group 2: Green synthesized Sodium silver fluoride (n-NaAgF) nanoparticle varnish (Figure 4) was applied with a sponge for three minutes over the specimens. After this, the varnish was kept in contact with the enamel surface of the specimens for 20 minutes. Then the samples were washed for 20 seconds with deionized water [23].
Figure 4: Preparation of Nano Sodium silver fluoride (n-NaAgF) from Camelia sinesis.
Group 3: Green synthesized hydroxyapatite nanoparticle varnish (n-HAP) (Figure 5) The application of the n-HAP varnish with a brush on the tooth surface and allowed to dry. The samples were then subjected to demineralization solution for 3 hours and remineralization solution (CCP-ACP) for 21 hours. This cycle of immersion in demineralization solution followed by immersion in remineralization solution was continued daily for one month with the solutions being made fresh every 3 days. The application of the n- HAP varnish was repeated every 10 days during this one month period [24].
Figure 5: Preparation of nano hydroxyapatite (n-HAP) from Camelia Sinesis.
Following the respective varnish treatment procedures, the surface microhardness test was done for the samples of the three groups and the values were determined (T3).
Statistical analysis: The power of the study was calculated using post-hoc in G power software to N=30. Statistics were calculated using the SPSS version 17 (IBM®, Chicago, IL, USA). The Shapiro-Wilk test confirmed the data distribution normality [25]. The surface microhardness test values and the surface microhardness recovery percentage were compared at three time intervals-T1, T2, and T3 with One-way ANOVA and also the Sidak Post-hoc test. Repeated measures ANOVA and the Tukey HSD Post hoc test were used for inter-group comparisons. The level of significance was set at 0.05.
Results
The T1, T2 and T3 microhardness test for each group is shown in (Table 1). At T1, the surface micro-hardness test values of all samples ranged from 262 to 389 (mean 330 ± 25.06) which was not statistically different among the groups (p=0.8). Surface microhardness test values of T2 ranged from 115.4 to 125.6 (mean 125.7 ± 16.5), were significantly lesser than the T1 surface micro-hardness values (p<0.05). The highest surface microhardness test values at T3 were observed in green synthesized sodium silver fluoride group (mean 192.6 ± 29.86) followed by that of green synthesized HAP (mean value:171.5 ± 20.66) while the control group had a mean value of 113.2 ± 19.3.
| T1 | T2 | T3 | |
|---|---|---|---|
| CONTROL | 333.1 ± 26.03 | 125.6 ± 17.2 | 113.2 ± 19.3 |
| n-NSF | 331.2 ± 26.3 | 115.4 ± 11.5 | 192.6 ± 29.86 |
| n-HAP | 330.4 ± 20.4 | 125.6 ± 11.8 | 171.5 ± 20.66 |
Table 1: Mean Surface microhardness values of the three groups at T1, T2 and T3. (Sidak Post hoc test).
Intergroup analysis with post hoc Tukey HSD test has been shown in Table 2. There was a significant difference between green synthesized sodium silver fluoride and green synthesized hydroxyapatite (p=0.001). The surface hardness recovery was observed in both the treatment groups but not in the control group [26]. The results of both the interventional groups revealed significant differences between the groups except for the control group (p=0.8).
| n-HAP | NSF | CONTROL | |
|---|---|---|---|
| n-HAP | p=0.2 | p<0.05 | p<0.001 |
| n-NSF | p<0.001 | p=0.7 | p<0.001 |
| Control | p<0.001 | p<0.001 | p=0.8 |
| level of significance p<0.05. | |||
Table 2: Comparison between groups of post-treatment surface microhardness values with repeated measure ANOVA and post-hoc Tukey HSD test
Discussion
The prevention of white spot lesion has been significance in orthodontics. Identification of early carious lesions and its prevention has led to development of many non-surgical methods 1920. Susceptibility of retention of plaque in orthodontic brackets is common. Diet, oral hygiene, frequency of eating has been the most important factors causing white spot lesions 21. In a study by Derks et al. he concluded that most orthodontists (95%) only provide oral hygiene instructions and around 50% provide fluoride mouth rinses. Hence all these factors increase the prevalence of white spot lesions among orthodontic patients and demands effective in office and at home oral hygiene [27]. This study demonstrates the efficacy of two nanoparticle varnishes namely green synthesized sodium Silver Fluoride (NSF) and green synthesized hydroxyapatite varnish for prevention of white spot lesions. Elements such as silver, gold, zinc oxide and chitosan have antimicrobial property against streptococcus mutans [23,24] which has led to development of formulations for anticaries activity [28].
Sondi et al. concluded that silver nanoparticles can cause structural changes and lead to bacterial cell death by penetrating the bacterial cell wall. Haghoo et al. had suggested that NSF could be a promising agent against Streptococcus Mutans and Streptococcus salivarius. Santos et al. compared Nano Silver Fluoride (NSF) with Silver Diamine Fluoride (SDF) for efficacy in arresting incipient caries in pedodontic patients. In their study, 66.7% of the lesions in teeth treated with NSF were arrested while only 34.7% treated with SDF were arrested. It was also found that SDF causes blackish discoloration of the teeth after remineralization due to deposition of silver particle penetration whereas this was less with Sodium silver fluoride varnish. In our study, the remineralization potential was highest with n-NSF group further attesting the efficacy of NSF in remineralization of enamel [29].
In this study we performed a green synthesis of n-NSF from Camelia Sinesis (White tea). Jose et al. showed that out of 21 plants used in their study, white tea (-87%) was found to have the highest anti-collagenase, anti-elastase and anti-oxidant activities followed by green tea (-47%), rose tincture (-41%) and lavender (-31%). Both the green tea and white tea have catechins like particles. In a study by rose et al. it has been found that the calcium in CPP-ACP binds to the plaque and doubles the availability of calcium and thus may aid in greater remineralization. Hence this was used in our study to increase the availability of calcium ions for remineralization. The results of this in vitro study revealed that green synthesized nano silver fluoride had a higher efficacy of remineralization compared to Nano hydroxyapatite varnish synthesized from Camelia sinesis. The effectiveness of remineralization of green synthesized n-silver fluoride and n-hydroxyapatite were similar with the other studies where silver fluoride varnish was compared with hydroxyapatite varnish 29,30,31. De Carvalho FG et al. had demonstrated similar remineralization results with nano hydroxyapatite. Aykildiz et al. compared the remineralizing capacity among sodium silver fluoride, SDF and NaF and they found sodium silver fluoride to have the highest remineralizing capacity which was similar to our results. Comar et al. concluded that fluoride (0.2% NaF) was better in remineralization compared n-HAP and n-HAP pastes where they had compared the prevention of demineralization rather than the remineralization efficacy [30]. Tschoppe P et al. concluded that nano hydroxyapatite was more effective than amine fluoride. However they had used n-HAP toothpastes with different formulations. This might have caused a difference in the results. They had also recommended usage of n-HAP as an alternative for candidates with fluorosis. Zhi QH et al. showed that both silver and fluoride were responsible for enamel remineralization and also demonstrated that silver can penetrate into those carious lesions and arrest the lesions [31]. The limitation of the current study is that it was an in vitro study, the effects of the varnish in an in vivo set up has yet to be studied to check for other possible beneficial/adverse effects of the varnishes and also validate the current results. Hence further studies to determine the long term effects in an in vivo setup of these two varnishes are necessary [32].
Conclusion
Green synthesized nano sodium silver fluoride and nano hydroxyapatite varnishes can be used for remineralization of incipient and white spot lesions that occur as a result of orthodontic treatment. The remineralization efficacy of nano silver fluoride was found to be higher than that of nano-hydroxyapatite.
References
- Johnson WW. Fundamentals of Operative Dentistry: A Contemporary Approach. J Prosthodont 2002; 11:224–225.
[Crossref]
- Ogaard B. White Spot Lesions during Orthodontic Treatment: Mechanisms and Fluoride Preventive Aspects. Seminars in Orthodontics 2008; 14:183–193.
- Hemadi AS, Huang R, Zhou Y, et al. Salivary proteins and microbiota as biomarkers for early childhood caries risk assessment. Int J Oral Sci 2017; 9:1.
- Sudjalim TR, Woods MG, Manton DJ, et al. Continuing professional development self-assessment quiz: Prevention of white spot lesions in orthodontic practice: a contemporary review. Aust Dent J 2006; 51:347–347.
[Crossref]
- Tufekci E, Dixon JS, Gunsolley JC, et al. Prevalence of White Spot Lesions during Orthodontic Treatment with Fixed Appliances. Case Med Res 2019.
- Kugel G, Arsenault P, Papas A, et al. Treatment modalities for caries management, including a new resin infiltration system. Compend Contin Educ Dent 2009; 3:1-10.
- Ogaard B, Rolla G, Arends J, et al. Orthodontic appliances and enamel demineralization. Am J Orthod Dentofacial Orthop 1988; 94:68–73.
- Kidd EAM, Fejerskov O. What constitutes dental caries? Histopathology of carious enamel and dentin related to the action of cariogenic biofilms. J Dent Res 2004; 83:35–38.
- Manji F, Fejerskov O, Nagelkerke NJ, et al. A random effects model for some epidemiological features of dental caries. Community Dent Oral Epidemiol 1991; 19:324–328.
- Larsen MJ. The nature of early caries lesions in enamel. J Dent Res 1986; 65:1030–1031.
- Weatherell JA, Robinson C, Hallsworth AS, et al. The Concept of Enamel Resistance. A Critical Review. Cariology Today; 223–230.
[Crossref]
- Bjørndal L, Thylstrup A. A structural analysis of approximal enamel caries lesions and subjacent dentin reactions. Eur J Oral Sci 1995; 103:25–31.
- Akin M, Basciftci FA. Can white spot lesions be treated effectively? Angle Orthod 2012; 82:770–775.
- Artun J, Thylstrup A. A 3-year clinical and SEM study of surface changes of carious enamel lesions after inactivation. Am J Orthod Dentofacial Orthop 1989; 95:327–333.
- Shahmoradi M, Hunter N, Swain M, et al. Efficacy of Fluoride Varnishes with Added Calcium Phosphate in the Protection of the Structural and Mechanical Properties of Enamel. Biomed Res Int 2017; 2017:7834905.
- Bonetti D, Clarkson JE. Fluoride Varnish for Caries Prevention: Efficacy and Implementation. Caries Res 2016; 50:45–49.
- Bayda S, Adeel M, Tuccinardi T, et al. The History of Nanoscience and Nanotechnology: From Chemical-Physical Applications to Nanomedicine. Molecules; 25.2019.
- Wang A, Wang W. Nanomaterials from Clay Minerals: A New Approach to Green Functional Materials. Elsevier 2019.
- Chen Z, Cao S, Wang H, et al. Biomimetic Remineralization of Demineralized Dentine Using Scaffold of CMC/ACP Nanocomplexes in an In Vitro Tooth Model of Deep Caries. PLOS ONE 2015; 10:0116553.
- Chaussain-Miller C, Fioretti F, Goldberg M, et al. The Role of Matrix Metalloproteinases (MMPs) in Human Caries. J Dent Res. 2006; 85:22–32.
- Fundamentals of Operative Dentistry: A Contemporary Approach.
- Derks A, Kuijpers-Jagtman AM, Frencken JE, et al. Caries preventive measures used in orthodontic practices: an evidence-based decision? Am J Orthod Dentofacial Orthop 2007; 132:165–170.
- Santos VE dos, dos Santos VE, Filho AV, et al. A New â??Silver-Bulletâ?? to treat caries in children – Nano Silver Fluoride: A randomised clinical trial. J Dent. 2014; 42:945–951.
- Hernandez-Sierra JF, Ruiz F, Pena DCC, et al. The antimicrobial sensitivity of Streptococcus mutans to nanoparticles of silver, zinc oxide, and gold. Nanomed: Nanotechnol, Biol Med 2008; 4:237–240.
- Kaabipour S, Hemmati S. A review on the green and sustainable synthesis of silver nanoparticles and one-dimensional silver nanostructures. Beilstein J Nanotechnol. 2021; 12: 102–136.
- Giulio MD, Di Giulio M, Di Bartolomeo S, et al. The Effect of a Silver Nanoparticle Polysaccharide System on Streptococcal and Saliva-Derived Biofilms. Int J Mol Sci 2013; 14: 13615–13625.
- Jose P, Sanjeev K, Sekar M, et al. Effect of Green and White Tea Pretreatment on Remineralization of Demineralized Dentin by CPP-ACFP-An Invitro Microhardness Analysis. J Clin Diagn Res 2016; 10:85–89.
- Rose RK. Binding Characteristics of Streptococcus mutans for Calcium and Casein Phosphopeptide. Creces 2000; 34:427–431.
- Tschoppe P, Zandim DL, Martus P, et al. Enamel and dentine remineralization by nano-hydroxyapatite toothpastes. J Dent 2011; 39:430–437.
- Comar LP, Souza BM, Gracindo LF, et al. Impact of experimental nano-HAP pastes on bovine enamel and dentin submitted to a pH cycling model. Braz Dent J 2013; 24:273–278.
- de Carvalho FG, Vieira BR, Santos RLD, et al. In vitro effects of nano-hydroxyapatite paste on initial enamel carious lesions. Pediatr Dent 2014; 36:85–89.
- Acton QA. Advances in Hydrofluoric Acid Research and Application: 2013 Edition: Scholarly Brief. Scholarly Editions, 2013.
Author Info
Department of Implantology, Rajiv Gandhi University of Health Sciences, Chennai, Tamil Nadu, IndiaCitation: Rama Raji Sankaranarayanan, Comparative Evaluation of Remineralization of White Spot Lesion in Nano Silver Fluoride and Nano Hydroxyapatite Varnish: An In vitro Study, J Res Med Dent Sci, 2022, 10 (9): 000-000.
Received: 28-Jun-2022, Manuscript No. JRMDS-22-50318; , Pre QC No. JRMDS-22-50318; Editor assigned: 30-Jun-2022, Pre QC No. JRMDS-22-50318; Reviewed: 14-Jul-2022, QC No. JRMDS-22-50318; Revised: 29-Aug-2022, Manuscript No. JRMDS-22-50318; Published: 05-Sep-2022