Research - (2020) Advances in Dental Surgery
Comparative Analysis of Salivary Homocysteine and Nitric Oxide Levels in Patients with Polycystic Ovarian Syndrome and Healthy Women-An in vivo Study
Srujana Hemmanur1, Iffat Nasim2* and Rizwana Aziz2
*Correspondence: Iffat Nasim, Department of Gynecology and Obstetrics, Govt RSRM Hospital, Stanley Medical College, Chennai, India, Email:
Abstract
Polycystic ovarian syndrome commonly known as PCOS is a collection of symptoms associated with a poor reproductive health. It is amongst the most common disorders affecting women of reproductive age. It is a multidimensional disorder with unknown aetiology. For diagnosis, currently Rotterdam criteria are widely being used. The aim of the study is to check for the correlation in the amounts of salivary homocysteine and nitric oxide in women with PCOS and healthy controls. The saliva samples were collected by spitting method from 20 women suffering from PCOS (test group) and 20 healthy women with no known disorders (control group). The saliva samples were tested for the presence of homocysteine and nitric oxide and their levels were quantified. In patients with PCOS, salivary homocysteine levels are significantly higher than the control group. However, the salivary nitric oxide levels are significantly lower in the test group when compared to the control group. The results obtained are like many serum and plasma analyses and salivary evaluation can be considered as a chairside technique for the evaluation of the same. However, an expanded study needs to be done to check for any confounding factors.
Keywords
Salivary, Homocysteine, Nitric oxide, PCOS, Syndrome
Introduction
Polycystic Ovarian syndrome commonly abbreviated as PCOS is a common disorder affecting women of reproductive age [1]. PCOS was first described in the US by Stein and Leventhal in 1935 and has been considered as one amongst the most common endocrine disorders of women [2]. The definition of PCOS given by the National Institutes of Health (NIH) in April 1990 involves (in order of importance): a) hyperandrogenism and/or hyperandrogenemia, 2) ovulatory dysfunction, and 3) exclusion of related disorders such as hyperprolactinemia, thyroid disorders, and congenital adrenal hyperplasia. The definition can be found consistent with the one given by the European Society for Human Reproduction and Embryology and the American Society for Reproductive Medicine in May 2003 in Rotterdam, The Netherlands [2]. Hence, it is a multidimensional disorder in association with endocrinological, reproductive, metabolic, and psychological alterations. It is seen highly amongst women of the reproductive age bracket, has an unknown etiology but a prevalence of 5-10% throughout the world [3]. Though the cause remains unknown, studies strongly indicate genetic components affected by gestational environment, factors modifying lifestyle or both behind the causation of PCOS [4]. Apositive familial history of around 17% of respondents with comorbidities including diabetes mellitus, hypertension, hypo/hyperthyroidism, gastrointestinal issues, and subfertility have been reported in asurvey conducted in 2018 [5]. Approximately 50-70% of the diagnosed cases have detectable Insulin resistance and hyperinsulinemia which stimulate androgen overproduction in ovaries and suppresses sex hormone binding globulin (SBHG) causing cerebrovascular and cardiovascular morbidities [2,6].
For the diagnosis, Rotterdam criteria is widely applied. The criteria suggest that at least two of the following: hyperandrogenism, ovulatory dysfunction and polycystic ovaries must exist. However, other potential causes of hyperandrogenism and dysfunctioning ovaries must be successfully ruled out [7]. The diagnosis can hence be thought to be made through an art of exclusion.
A meta-analysis of 21 studies concluded that dysfunction of endothelium was quite evident in women suffering with PCOS. Nitric oxide and homocysteine in altered concentrations have been found to be associated with endothelial dysfunction [8]. Nitric oxide, a free radical gas molecule, involved in various physiological and pathological processes, is reduced biologically owing to an increased level of superoxide radicals. A limited number of studies indicate that the levels of Nitric Oxide in women with PCOS is questionable [3]. Homocysteine, an amino acid formed by the conversion of methionine to cysteine shows elevated serum concentration in several studies conducted in women with polycystic ovarian syndrome versus healthy controls [3,9,10]. Recently, a study showed the correlation of the serum and salivary levels of homocysteine in patients with Ischaemic Heart Disease (IHD), which helped form the backdrop of the current study [11].
We have numerous highly cited publications on well-designed clinical trials and lab studies [12- 27]. This has provided the right platforms for us to pursue the current study. Our aim was to check whether altered amounts of homocysteine and nitric oxide can be found in the salivary samples collected from women with PCOS as compared to healthy women.
Materials and Methods
Approval from the Institutional Ethics Committee was obtained before conducting the research. The patients were informed about the procedure, an informed consent was taken, and their identity was promised to not be disclosed.
Inclusion criteria
Patients diagnosed with PCOS and not under medication.
Patients with no other known endocrinological or metabolic disorders.
Age: 20 to 30 years.
Exclusion criteria
Patients under medication for PCOS.
Diagnosed with other known endocrinological or metabolic disorders in association with PCOS.
Age beyond 30 years.
5 ml of saliva samples by spitting method was collected in a test tube and kept in refrigeration at 4℃ till the sample wasn’t tested. A total of 40 samples in which 20 samples from the patients diagnosed with PCOS and the remaining 20 samples from healthy women were collected. The collected samples were tested within 24 hours of collection.
Estimation of homocysteine
To 5 μl of saliva sample 20 ml of borate buffer (125 mM boric acid, 4 mM EDTA) was added to make the final volume to 25 μl. 10% (V/V) of tri-n-butyl phosphine in dimethylformamide was added to the sample and vortexed. The resulting mixture was incubated at 4°C for 30 min. Deproteinization was done with 25 μl 10% (W/V) TCA, followed by vortexing and centrifugation at 3000 RPM for 15 min at 4°C. 20 μl of clear supernatant taken in a fresh tube, 4 μl of(1.55N)NAOH was added and vortexed. This tube was kept at 4°C for 10 min and a 50 μl of borate buffer was added and vortexed. Finally, 20 ml of SBDF (ammonium 7-flurobenzo-2- oxa-1, 3-diazole-4sulfonate 1mg/ml in 125 mM boric acid) was added and incubated at 60°C for one hour. The incubation sample was kept at ambient temperature for immediate processing. Standards and controls were prepared simultaneously in a similar manner.
HPLC Chromatography
The sample was injected into a column equilibrated with mobile phase at a flow rate of 1.5 ml per min. The column equilibration time was 15 minutes at ambient temperature. The retention time of each sample was calculated by using standards and calibrators and a calibration curve was plotted for different concentrations. Stationary phase: Octadecyl silane (ODS) 250X4.6 mm column, Mobile phase: 4% acetonitrile in potassium dihydrogen orthophosphate (0.2 M, pH: 2.1), Detector: fluorescent with Ex/Em being 385 nm/515 nm.
Estimation of nitric oxide
In this method, nitrite is first treated with a diazotizing reagent, e.g., sulfanilamide (SA), in acidic media to form a transient diazonium salt. This intermediate was then allowed to react with a couplingr eagent, N-naphthyl-ethylenediamine (NED), to form a stable azo compound. The intense purple color of the product allows nitrite assay with high sensitivity. The absorbance of this adduct at 540 nm is linearly proportional to the nitrite concentration in the sample. Salivary samples (50 μl) were transferred to a 96-well enzyme-linked immunosorbent assay plate. Using a multichannel pipettor, 50 μl of the sulfanilamide solution (1% sulfanilamide in 5% phosphoric acid) followed by 50 μl of the naphthyl ethylene.
In this method, nitrite was first treated with a diazotizing reagent, e.g., sulfanilamide (SA), in acidic media to form a transient diazonium salt. This intermediate is then allowed to react with a coupling reagent, N-naphthyl-ethylenediamine (NED), to form a stable azo compound. The intense purple color of the product allows nitrite assay with high sensitivity. The absorbance of this adduct at 540 nm is linearly proportional to the nitrite concentration in the sample. Salivary samples (50 μl) were transferred to a 96-well enzyme-linked immunosorbent assay plate. Using a multichannel pipettor, 50 μl of the sulfanilamide solution (1% sulfanilamide in 5% phosphoric acid) followed by 50 μl of the naphthyl ethylenediamine solution (0.1% N-naphthyl ethylenediamine) was dispensed to all experimental samples. The samples were incubated at room temperature for 5–10 min. A purple color was observed, and its optical density was measured using a plate reader with 540 nm filter.
Statistical analysis
All the data was entered into Microsoft Excel 2010. Descriptive statistics were expressed as mean ± standard deviation (SD) for each group for salivary homocysteine and salivary nitric oxide (μM/ml). Two groups (Women with PCOS and Healthy subjects) were compared for Salivary Homocysteine and Salivary Nitric Oxide (μM/ml) by Independent ‘t’ Test.
For All the above test p value is considered statistically significant when it was <0.05. The software used was SPSS (Statistical package for Social Sciences) version 17.
Results and Discussion
Descriptive statistics are mentioned in Table 1. Group 1 Women with PCOS (27.76000 ± 5.083347) and Group 2 Healthy subjects (40.33000 ± 4.926096). X- axis represents the health status of the patient while Y-axis depicts the amount of salivary nitric oxide. It can be inferred that salivary nitric oxide is significantly lower in women with PCOS when compared to the healthy subjects (p value<0.001 with t= -7.941; df=38; Independent ‘t’ test). The current study helps to establish a positive correlation between the levels of salivary homocysteine and nitric oxide in patients with PCOS and healthy women (Figures 1 and 2).
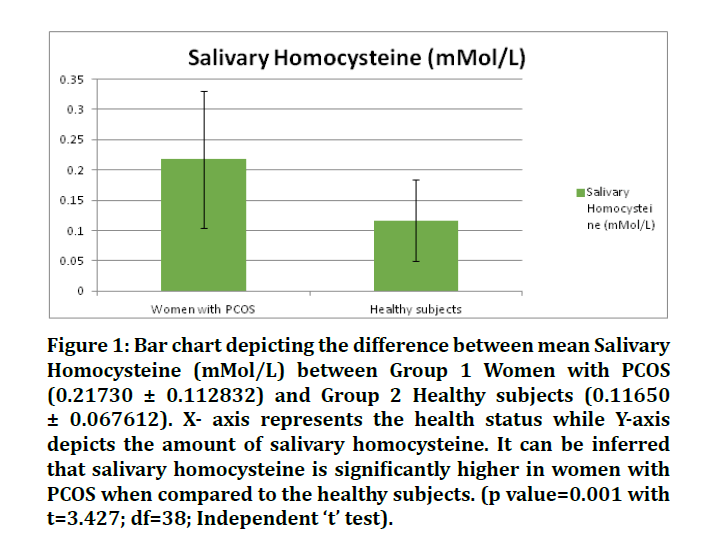
Figure 1: Bar chart depicting the difference between mean Salivary Homocysteine (mMol/L) between Group 1 Women with PCOS (0.21730 ± 0.112832) and Group 2 Healthy subjects (0.11650 ± 0.067612). X- axis represents the health status while Y-axis depicts the amount of salivary homocysteine. It can be inferred that salivary homocysteine is significantly higher in women with PCOS when compared to the healthy subjects. (p value=0.001 with t=3.427; df=38; Independent ‘t’ test).
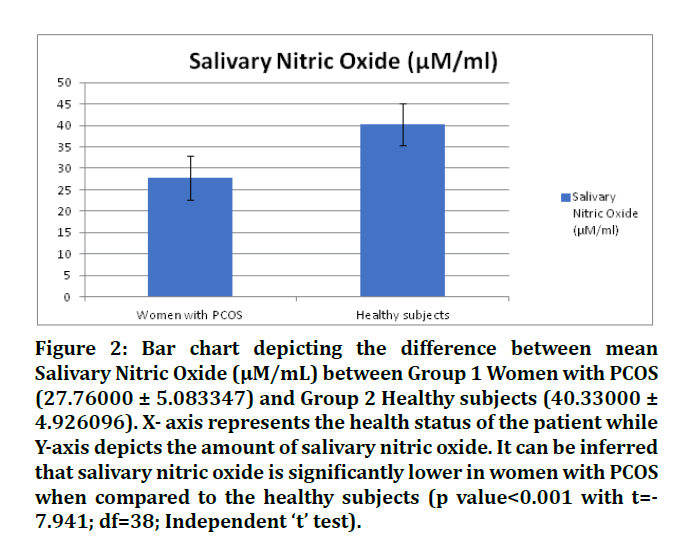
Figure 2: Bar chart depicting the difference between mean Salivary Nitric Oxide (μM/mL) between Group 1 Women with PCOS (27.76000 ± 5.083347) and Group 2 Healthy subjects (40.33000 ± 4.926096). X- axis represents the health status of the patient while Y-axis depicts the amount of salivary nitric oxide. It can be inferred that salivary nitric oxide is significantly lower in women with PCOS when compared to the healthy subjects (p value<0.001 with t=- 7.941; df=38; Independent ‘t’ test).
| Descriptive Statistics | ||||||
|---|---|---|---|---|---|---|
| Group | N | Minimum | Maximum | Mean | Std. Deviation | |
| Women with PCOS | Salivary Homocysteine (mMol/L) | 20 | 0.02 | 0.45 | 0.2173 | 0.112832 |
| Healthy subjects | Salivary Homocysteine (mMol/L) | 20 | 0.01 | 0.23 | 0.1165 | 0.067612 |
| Women with PCOS | Salivary Nitric Oxide (µM/ml) | 20 | 14.5 | 39 | 27.76 | 5.083347 |
| Healthy subjects | Salivary Nitric Oxide (µM/ml) | 20 | 32 | 49.2 | 40.33 | 4.926096 |
Table 1: Descriptive Statistics of Salivary Homocysteine (mMol/L) and Nitric Oxide (µM/ml) among two groups (i.e Women with PCOS and healthy subjects). It can be inferred that the mean of salivary homocysteine levels in PCOS patients is greater than that in healthy women. However, the mean of salivary nitric oxide levels in patients with PCOS is less than that of healthy women.
Women with PCOS are seen to have statistically significant greater levels of salivary homocysteine and lesser levels of nitric oxide than the control group (i.e. healthy women). Similar results however through the analysis of serum and plasma were obtained by several authors [3,9,10,28-33]. However, no difference in the levels of serum nitric oxide in PCOS and control group was reported by Nacul et al. [34]. Similarly, Mancini et al. reported no significant difference in the serum homocysteine levels in women with PCOS and control group [35]. Another study that compared the serum levels of homocysteine and nitric oxide in PCOS and healthy patients with and/or without periodontal disease found a highly significant correlation amongst the test and control groups [36].
Salivaa unique biological fluid with a wide spectrum of components that include proteins, hormones, nucleic acids, polypeptides and electrolytes [37]. Saliva is gaining importance in recent years and is considered a diagnostic tool for various reasons. Saliva is equivalent to the serum of the body as it reflects the physiological state of the body that include variations on hormonal, emotional, nutritional, and metabolic levels [38]. The values of the components found in serum can thus be directly correlated to salivary secretion of the same. Saliva is being considered as a valuable tool for diagnosis as the procedure is simple non-invasive chairside collection of voluminous samples and is a cost-effective method for mass screening of innumerable components. A study conducted on 33 ischaemic heart disorder patients concluded a significant positive correlation between the salivary and serum levels of homocysteine, ghrelin and obestatin and thus formed the backbone of the current study [11].
Homocysteine is found to be significantly higher in women with PCOS as compared to control group. It plays an established role in cardiovascular morbidity and mortality as it has prothrombotic and atherogenic properties. The elevated homocysteine levels thus, might play a crucial role in the increased risk of cardiovascular diseases [39] in women suffering from PCOS. High homocysteine levels are independently considered as a risk factor for cardiovascular diseases as the increased oxidative stress in vascular endothelium and activation of platelet aggregation leading to dysfunction of endothelium. Another important finding is that increased homocysteine levels causes decreased availability of nitric oxide in serum that is an early marker of vascular disease. Nitric oxide’s contribution to vessel homeostasis is by the growth, platelet aggregation and leukocyte adhesion to endothelium and inhibition of vascular smooth muscle contraction [40]. The reduced bioavailability of nitric oxide and increased oxidative stress both cause endothelial dysfunction [41] which again directs towards the increased risk of cardiovascular diseases in women with PCOS as a comorbidity.
Conclusion
The current study found a statistically significant association between salivary homocysteine and nitric oxide levels of patients with PCOS and control group. This evaluation of salivary homocysteine and nitric oxide levels in women with PCOS and healthy subjects could help formulate a chairside test for confirmation of the syndrome along with proper history. Also, the women with a greater risk of cardiovascular diseases could be helped by conforming their lifestyle and prevention or at least delay the occurrence of cardiovascular morbidity. However, the study must be done on a larger population scale to confirm the results and the confounding factors, if any.
Conflict of Interests
Nil.
References
- Wolf WM, Wattick RA, Kinkade ON, et al. Geographical prevalence of polycystic ovary syndrome as determined by region and race/ethnicity. Int J Environmental Res Public Health 2018; 15:2589.
- Azziz R, Marin C, Hoq L, et al. Health care-related economic burden of the polycystic ovary syndrome during the reproductive life span. J Clin Endocrinol Metabol 2005; 90:4650–4658.
- Kandasamy S, Inmozhi Sivagamasundari R, Bupathy A, et al. The plasma nitric oxide and homocysteine levels and their association with insulin resistance in south Indian women with polycystic ovary syndrome. Int Med J Exp Clin Res 2016; 4:4829.
- Norman, R. J. et al. (2007) ‘Polycystic ovary syndrome’, The Lancet. Elsevier, 370(9588), pp. 685–697.
- Rao M, Broughton S. An exploratory survey to estimate prevalence, and perceived knowledge of Polycystic ovary syndrome (PCOS) in young adults. Endocrinol Metabol Syndrome 2018; 7.
- Baptiste CG, Battista MC, Trottier A, et al. Insulin and hyperandrogenism in women with polycystic ovary syndrome. J Steroid Biochem Molecular Biol 2010; 122:42–52.
- Mortada R, Williams T. Metabolic syndrome: Polycystic ovary syndrome. FP Essentials 2015; 435:30–42.
- Lai WKC, Kan MY. Homocysteine-induced endothelial dysfunction. Annals Nutrition Metabol 2015; 67:1–12.
- Salehpour S. Evaluation of homocysteine levels in patients with polycystic ovarian syndrome. Int J Fertility Sterility 2011; 4:168–171.
- Saadeh N, Alfaqih MA, Mansour H, et al. Serum homocysteine is associated with polycystic ovarian syndrome in Jordan. Biomed Reports 2018; 9:439–445.
- Kilic N, Dagli N, Aydin S, et al. Saliva/serum ghrelin, obestatin and homocysteine levels in patients with ischaemic heart disease. Cardiovascular J Africa 2017; 28:159–164.
- Govindaraju L, Neelakantan P, Gutmann JL. Effect of root canal irrigating solutions on the compressive strength of tricalcium silicate cements. Clin Oral Investigations 2017; 21:567–571.
- Azeem RA, Sureshbabu NM. Clinical performance of direct versus indirect composite restorations in posterior teeth: A systematic review. J Conservative Dent 2018; 21:2–9.
- Jenarthanan S, Subbarao C. Comparative evaluation of the efficacy of diclofenac sodium administered using different delivery routes in the management of endodontic pain: A randomized controlled clinical trial. J Conservative Dent 2018; 21:297–301.
- Manohar MP, Sharma S. A survey of the knowledge, attitude, and awareness about the principal choice of intracanal medicaments among the general dental practitioners and nonendodontic specialists. Indian J Dent Res 2018; 29:716–720.
- Nandakumar M, Nasim I. Comparative evaluation of grape seed and cranberry extracts in preventing enamel erosion: An optical emission spectrometric analysis. J Conservative Dent 2018; 21:516–520.
- Teja KV, Ramesh S, Priya V. Regulation of matrix metalloproteinase-3 gene expression in inflammation: A molecular study. J Conservative Dent 2018; 21:592–596.
- Janani K, Sandhya R. A survey on skills for cone beam computed tomography interpretation among endodontists for endodontic treatment procedure. Indian J Dent Res 2019; 30:834–838.
- Khandelwal A, Palanivelu A. Correlation between dental caries and salivary albumin in adult population in Chennai: An In Vivo Study. Brazilian Dent Sci 2019; 22:228–233.
- Malli Sureshbabu N, Selvarasu K, Nandakumar M, et al. Concentrated growth factors as an ingenious biomaterial in regeneration of bony defects after periapical surgery: A report of two cases. Case reports Dent 2019; 2019:7046203.
- Poorni S, Srinivasan MR, Nivedhitha MS. Probiotic strains in caries prevention: A systematic review. J Conservative Dent 2019; 22:123–128.
- Rajakeerthi R, Ms N. Natural product as the storage medium for an avulsed tooth–A systematic review. Cumhuriyet Dent J 2019; 22: 249–256.
- Rajendran R, Kunjusankaran RN, Sandhya R, et al. Comparative evaluation of remineralizing potential of a paste containing bioactive glass and a topical cream containing casein phosphopeptide-Amorphous calcium phosphate: An in Vitro study. Pesquisa Brasileira Odontopediatria Clin Integrada 2019; 19:1–10.
- Ramarao S, Sathyanarayanan U. CRA Grid-A preliminary development and calibration of a paper-based objectivization of caries risk assessment in undergraduate dental education. J Conservative Dent 2019; 22:185–190.
- Siddique R, Nivedhitha, MS. Effectiveness of rotary and reciprocating systems on microbial reduction: A systematic review. J Conservative Dent 2019; 22:114–122.
- Siddique R, Sureshbabu NM, Somasundaram J, et al. Qualitative and quantitative analysis of precipitate formation following interaction of chlorhexidine with sodium hypochlorite, neem, and tulsi. J Conservative Dent 2019; 22:40–47.
- Siddique R, Nivedhitha MS, Jacob B. Quantitative analysis for detection of toxic elements in various irrigants, their combination (precipitate), and para-chloroaniline: An inductively coupled plasma mass spectrometry study. J Conservative Dent 2019; 22:344–350.
- Bayraktar F, Dereli D, Özgen AG, et al. Plasma homocysteine levels in polycystic ovary syndrome and congenital adrenal hyperplasia. Endocrine J 2004; 51:601–608.
- Yilmaz M, Bi˙ ri˙ A, Bukan N, et al. Levels of lipoprotein and homocysteine in non-obese and obese patients with polycystic ovary syndrome. Gynecol Endocrinol 2005; 20:258–263.
- Badawy A, State O, Abd El Gawad SS, et al. Plasma homocysteine and polycystic ovary syndrome: the missed link. European J Obstetr Gynecol Reproductive Biol 2007; 131:68–72.
- de la Calle M, Gallardo T, Diestro MD, et al. Increased homocysteine levels in polycystic ovary syndrome. Med Clin 2007; 129:292–294.
- Maleedhu P, Vijayabhaskar M, Sharma SS, et al. Status of homocysteine in polycystic ovary syndrome (PCOS). J Clin Diagnostic Res 2014; 8:31–33.
- Meng Y, Chen X, Peng Z, et al. (2016) Association between high serum homocysteine levels and biochemical characteristics in women with polycystic ovarian syndrome: A systematic review and meta-analysis. PloS One 2016; 11:e0157389.
- Nacul AP, Andrade CD, Schwarz P, et al. Nitric oxide and fibrinogen in polycystic ovary syndrome: Associations with insulin resistance and obesity. Eur J Obstetr Gynecol Reproductive Biol 2007; 133:191–196.
- Mancini F, Cianciosi A, Reggiani GM, et al. Endothelial function and its relationship to leptin, homocysteine, and insulin resistance in lean and overweight eumenorrheic women and PCOS patients: A pilot study. Fertility Sterility 2009; 91:2537–2544.
- Hameed DJ, Ahmed MAA. Evaluation of serum homocysteine and nitric oxide levels in women with polycystic ovarian syndrome and periodontal diseases. Tikrit J Dent Sci 2017; 5:57–65.
- Sindhu S, Jagannathan N. Saliva: A cutting edge in diagnostic procedures. J Oral Diseases 2014; 2014.
- Lee YH, Wong DT. Saliva: An emerging biofluid for early detection of diseases. Am J Dent 2009; 22:241.
- Tyagi N, Sedoris KC, Steed M, et al. Mechanisms of homocysteine-induced oxidative stress. Am J Physiol Heart Circulatory Physiol 2005; 289:H2649–H2656.
- Willis GR, Udiawar M, Evans WD. Detailed characterisation of circulatory nitric oxide and free radical indices—is there evidence for abnormal cardiovascular homeostasis in young women with polycystic ovary syndrome? J Obstetr 2014; 121:1596-1603.
- Li M, Qian M, Kyler K, et al. Endothelial–vascular smooth muscle cells interactions in atherosclerosis. Frontiers Cardiovascular Med 2018; 5:151.
Author Info
Srujana Hemmanur1, Iffat Nasim2* and Rizwana Aziz2
1Department of Conservative dentistry and Endodontics, Saveetha Institute of Medical and Technical Sciences, Saveetha University, Chennai, India2Department of Gynecology and Obstetrics, Govt RSRM Hospital, Stanley Medical College, Chennai, India
Citation: Srujana Hemmanur, Iffat Nasim, Rizwana Aziz, Comparative Analysis of Salivary Homocysteine and Nitric Oxide Levels in Patients with Polycystic Ovarian Syndrome and Healthy Women-An in vivo Study, J Res Med Dent Sci, 2020, 8 (7): 240-245.
Received: 16-Sep-2020 Accepted: 02-Nov-2020 Published: 09-Nov-2020
