Research - (2023) Volume 11, Issue 1
Clinical Features and Associated Comorbidities in 200 Cases of COVID-19 in Peshawar, Pakistan
Ihteshamul Haq1, Shah Faisal2*, Abdullah3, Muhammad Romman4, Naumana Rhman5, Misbah Uddin6, Ghazala Zarin Afridi7, Sajjad Ali Shah2, Faheem Anwar8, Fazli Zahir9, Fatima Syed10, Naveed Iqbal9 and Shahzad Shoukat11
*Correspondence: Shah Faisal, Department of Biotechnology, Bacha Khan University, Charsadda, KPK, Pakistan, Email:
Abstract
SARS-CoV-2 is an emerging pathogen first reported in China and rapidly spreads throughout the world. The current study focused on the prevalence of symptoms, leukocytosis, leukocytopenia, thrombocytopenia, and comorbidity in patients resulted positive for COVID-19. About 200 patients were enrolled in the study first screened by ICT and then confirmed by RT-PCR. All the patients have symptoms fever, sore throat and dry cough. However 180 (90%) patients experienced tiredness, aches and pain all over the body in 193 (96%), diarrhea in 160 (80%), conjunctivitis in 64 (32%), headache in 177 (88%), loss of taste and smell in 49 (24%), a rash on skin, or discoloration of fingers or toes in 54 (27%), shortness of breath in 12 (8%), chest pain or pressure in 16 (8%), loss of speech and movement in 4 (2%) patients. 78 (39%) patients were found having co-infections including 23 (29.5%) patients have liver infection, 14 (17.9%) were kidney patients, 16 (20.5%) were heart patients and 25 (32%) were diabetic patients. Leukocytosis were found in 76 (97%) out 78 comorbid patients, while in only 2 (2.6%) non-comorbid patients had leukocytosis. Leukopenia was detected in 122 (61%) non-morbid patients out of 200 COVID-19 patients. Thrombocytopenia was detected in 118 (59%) COVID-19 patients. The patients with previous cardiovascular diseases and other comorbid conditions may face greater risk of developing the disease into severe form. 51-60 years of individuals are at high risk of getting infection. The hematologic changes are associated with COVID-19 includes thrombocytopenia, leukocytosis, and leukocytopenia.
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Keywords
SARS-COV-2, Clinical features, Comorbidities, COVID-19, RT-PCR, Leukocytosis
Introduction
Corona virus infection 2019 (COVID-19) is caused by Sever Acute Respiratory Syndrome Coronavirus-2 (SARSCoV- 2). Most of the coronaviruses are host specific [1]. The rapid transmission rate (R0 value) became a serious threat to human population. The world experienced the two serious outbreaks of SARS [2] and MERS [3] in 2003 and 2012, respectively. In December 2019, an outbreak arose in china and rapidly spread throughout the world. A cohort study on 36 children revealed the common symptoms including Fever (6%), dry cough (19%) and high elevated temperature of the body. Moreover, 31% patients have decreased lymphocytes while 19% have leucopenia [4]. Leukopenia, eosinophil cytopenia and lymphocytopenia are more common in patients with COVID-19 than non COVID-19 patients [4]. COVID-19 positive patients showed relatively lower absolute white blood cell (WBC), lymphocyte and eosinophil count. However, leukopenia was developed in 2 patients out of 10 and lymphocytopenia were also reported in 2 patients [4]. A 27-year-old lady in Wuhan china with history of fever and cough showed leucopenia while later she was detected positive for COVID-19 [4,5]. Environmental pollution, smoking and comorbid condition (Cardiorespiratory illness, hypertension, and diabetes mellitus) likely increase the fatality rate of COVID-19 [5,6]. COVID-19 can result in Leukopenia, thrombocytopenia, myocarditis and pneumonia. Globally the COVID-19 fatality rates are 5.6 to 15.2% [7], with a greater risk of mortality in old population and those having comorbid condition like hypertension and diabetes mellitus [8]. An analysis of 46,248 cases revealed the most prevalent comorbidities that is 17 % hypertension, 8% diabetes mellitus, 5 % cardiovascular disease and 2% respiratory morbidity [9]. A total of 6 studies involving 1527 patients revealed the percentages of hypertension, cardiovascular disease and diabetes mellitus with COVID-19 were 17.1%, 16.4% and 9.7%, respectively. It was also observed that the incidence of cardiovascular diseases diabetes and hypertension were about 3 folds, 2 folds and 2 folds respectively, higher in severe cases than non-severe cases [10]. Liver damage with COVID-19 might be directly cause by viral infection of liver cells. Patients with 2-10% diarhorrae and detection of viral RNA in stools and blood samples [11] implicate the possibility of viral exposure in liver. This seems the patients with COVID-19 to have higher rates of liver dysfunction [12]. A study revealed hepatitis B infection in 23 (2.1%) patients with COVID-19, Abnormal LFT and elevated aminotransferase, alanine aminotransferase and bilirubin were also noted [13]. Existing data suggest that prevalence of liver injury is more insevere cases than mild cases of COVID-19 [14].The current study aims to address the prevalence of symptoms, leukocytosis, leukocytopenia and thrombocytopenia and comorbidity in patients positive for COVID-19.
Methods and Materials
The current study was performed at Hayatabad Medical Complex, Peshawar, Pakistan and in Department of Biotechnology and Genetics Engineering Hazara University Mansehra, Pakistan. Ethical approval for the study was issued by Hazara University Mansehra Pakistan.
Enrollment of patients
A total of 200 suspected patients were enrolled in the study based on the onset of sign and symptoms related to COVID-19. Every suspected patient was asked for the symptoms before testing for COVID-19. Moreover, the comorbidities were also addressed by examining the patient’s history. The patients were divided in categories with respect to their age to ease in finding the comorbidities.
ICT Assay for COVID-19
Total 5ml blood was taken from everyone in two separate EDTA tubes and was stored for further analysis. To isolate serum from blood samples, 2ml blood was transferred to Eppendorf tubes and was centrifuged at 2000rpmfor2min. The supernatant was transferred throughmicro-pipette in a separate tube and stored for further analysis.
The COVID rapid test was performed using serum. One drop of serum (approximately 30μl) was transferred on the test strips and one drop of buffer (approximately 40μl) was added. After few minutes colored line was appeared and results were recorded.
Appearance of two distinct red lines, one line in the control region (C) and second in test region (T), showed positive results. Only one red line appearance in the control region (C) not in test region indicated negative results. The red color concentration in the test line region (T) varies depending on the conception of Covid-19 antibodies in the specimen. Therefore, any shade of red in the test region (T) was considered positive.
RT-PCR for the detection and confirmation of SARSCoV- 2
Throat swab sample were taken from patients screened positive for COVID-19 by ICT strip for the extraction of viral RNA. Soon after collection the throat swab were kept in 150 μL of viral preserving solution in a collection tube. Respiratory sample RNA isolation kit (Zhongzhi, Wuhan, China) were used for the extraction of viral RNA from throat swab and this process were done within two hours of sample collection. Cell lysate of 40 μL were immediately transferred to collection tube and start vortexing for 10 sec. The collection tube is leftover for 10 min at room temperature and centrifuged at 1000 rpm for 5 min. Suspension were obtained and used for RTPCR to detect the viral RNA of SARS-CoV-2. As shown in Figure 1 the two target genes ORF1aband nucleocapsid protein (N), were amplified and then tested at the same time in the RT-PCR process.
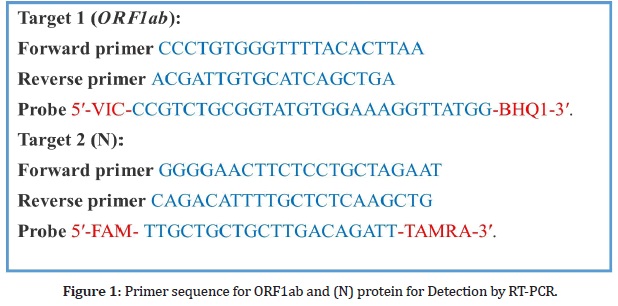
Figure 1: Primer sequence for ORF1ab and (N) protein for Detection by RT-PCR.
SARS-CoV-2 nucleic acid detection kit was used in RTPCR according to the company protocol (Shanghai biogerm Medical Technology Co Ltd). The reaction mixture contains 4 μL solution of enzyme, 12 μL buffer, 3 μL of diethyl pyrocarbonate–treated water,4 μL solution of primer Probe and RNA template of 2 μL.
The conditions which is maintained for the assay of RTPCR are the following; 50 °C for 15 min and 95 °C for 5 min were maintained at incubation step, denaturation were performed at 94 °C for 15 sec, 55 °C for 45 sec were the conditions for extension step. Fluorescence signal were collected at the same conditions that were maintained for extension.
Ct-value or cycle threshold value of less than 37 is considered positive while 40 or more Ct-value was defined as a negative test result. The recommendations of National Institute for Viral Disease Control and Prevention China for diagnosis were strictly followed.
Complete blood count (CBC)
The CBC was determined for patients undergoing hypovolumetric shock condition. The blood profile was determined by ALERE-380 blood cell counter machine. The main components of blood such as WBC, Platelets, and Lymphocytes values were recorded from all suspected patients.
Results
The enrolled 200 patients were screened for COVID-19 by ICT strip and were confirmed by the RT PCR. Agewise distribution of the patients were found positive for COVID-19 by ICT strip and confirmed by RT-PCR are; 12 (6%), 21 (10.5%), 33 (16.5%), 43 (21.5%), 80 (40%) and 11 (5.5%) in 1-20, 21-30, 31-40, 41-50, 51-60 and 61-70 years of age respectively as shown in Figure 2.
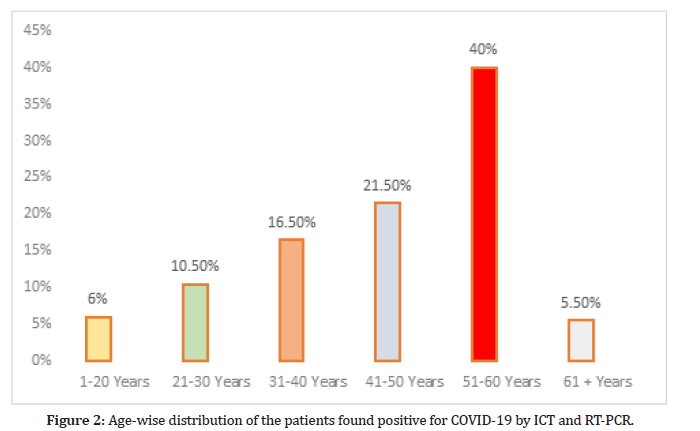
Figure 2:Age-wise distribution of the patients found positive for COVID-19 by ICT and RT-PCR.
Prevalence of symptoms
According to the questionnaire all the patients experienced fever, sore throat and dry cough. However 180 (90%) of patients experienced tiredness, aches and pain all over the body in 193 (96%), diarrhea in 160 (80%), conjunctivitis in 64 (32%), headache in 177 (88%), loss of taste or smell in 49 (24%), a rash on skin, or discoloration of fingers or toes in 54 (27%), difficulty in breathing or shortness of breath in 12 (8%), chest pain or pressure in 16 (8%), loss of speech or movement in 4 (2%) patients as shown in Figure 3. The percentages were separately find out in total for each symptom, however there are some patients who have many symptoms at a time.
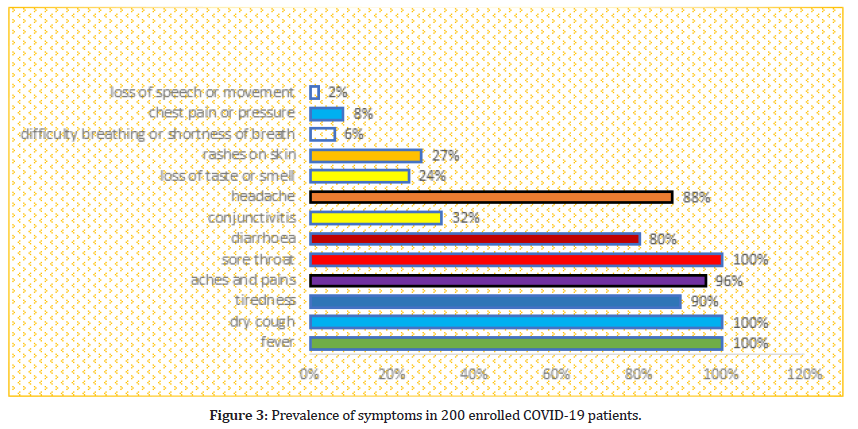
Figure 3:Prevalence of symptoms in 200 enrolled COVID-19 patients.
Prevalence of comorbidities/coinfections
The 200 patients were found positive for COVID-19 by RT-PCR were further examine for co-infection. 78 (39%) Patients out of 200 were found to have co-infection in which 23 (29.5%) patients have liver infection (11 (14.2%) were HBs and 12 (15.4%) were HCV positive), 14 (17.9%) were kidney patients, 16 (20.5%) were heart patients and 25 (32%) were diabetic patients.
The age-wise distribution of comorbidities along with COVID-19 are HBs; 0, 1 (1.2%), 1 (1.2%), 3 (3.8%), 5 (6.4%), 1 (1.2%), HCV; 0, 0, 2 (2.6%), 5 (6.4%), 3 (3.8%), 2 (2.6%), Kidney patients; 1 (1.2%), 2 (2.6%), 3 (3.8%), 4 (5.1%), 3 (3.8%), 1 (1.2%), heart patients; 0, 0, 0, 6 (7.7%), 7 (8.9%), 3 (3.8%), diabetic patients; 0, 0, 3 (3.8%), 6 (7.7%), 11 (14.1%) and 5 (6.4%)in 1-20, 21-30, 31-40, 41-50, 51-60 and 61-70 years of age respectively, as shown in Table 1.
| Age years | Number of Patients | Total coinfection | HBs | HCV | Kidney patients | Heart patients | Diabetic patient |
|---|---|---|---|---|---|---|---|
| 1-20 | 12.60% | 2.10% | 0% | 0% | 1.1.2% | 0% | 0% |
| 21-30 | 21.10.5% | 6.30% | 1.1.2% | 0% | 2.2.6% | 0% | 0% |
| 31-40 | 33.16.5% | 10.50% | 1.1.2% | 2.2.6% | 3.3.8% | 0% | 3.3.8% |
| 41-50 | 43.21.5% | 18.90% | 3.3.8% | 5.6.4% | 4.5.1% | 6.7.7% | 6.7.7% |
| 51-60 | 80.40% | 30.15% | 5.6.4% | 3.3.8% | 3.3.8% | 7.8.9% | 11.14.1% |
| 61 + | 11.5.5% | 12.60% | 1.1.2% | 2.2.6% | 1.1.2% | 3.3.8% | 5.6.4% |
| Total | 200 | 78 (39%) | 11 (14.2%) | 12 (15.4%) | 14 (17.9%) | 16 (20.5%) | 25 (32%) |
Table 1: Age-wise prevalence of comorbidities/coinfections.
Prevalence of leukocytosis, leukopenia and thrombocytopenia
Leukocytosis were found in 76 (97%) out 78 comorbid patients, while in only 2 (2.6%) non comorbid patients have leukocytosis. Leukopenia were detected in 122 (61%) non morbid patients out of 200 COVID-19 patients. Thrombocytopenia were detected in 118 (59%) COVID-19 patients (Figure 4).
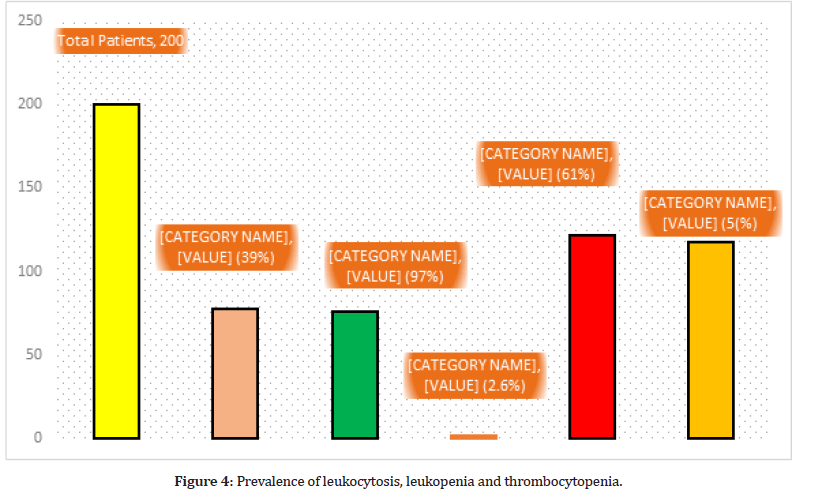
Figure 4:Prevalence of leukocytosis, leukopenia and thrombocytopenia.
Discussion
200 patients were enrolled in the study and screened for COVID-19 and then confirmed by RT-PCR. As the percentage of COVID-19 patients were found high 80% in the age 51-60 this is due to the weak immune system and more prevalence of comorbidity in this group. Globally the COVID-19 fatality rates are 5.6 to 15.2% [7], with a greater risk of mortality in old population and those having comorbid condition like hypertension and diabetes mellitus [8]. The lowest number of cases were detected in age group 61+ which 5.5%. This is because the patients low number in this group. Most of the patients developed symptoms of fever, throat, dry cough, shortness in breath, headache etc. the percentages are fever, sore throat and dry cough 100%, 90% of patients experienced tiredness, aches and pain all over the body in 96%, diarrhea in 80%, conjunctivitis in 32%, headache in 88%, loss of taste or smell in 24%, a rash on skin, or discoloration of fingers or toes in 27%, difficulty breathing or shortness of breath in 8%, chest pain or pressure in 8%, loss of speech or movement in 2% patients. Same findings were also observed by [4,15]. The percentages were separately finding out in total for each symptom; however there are some patients who have many symptoms at a time.
39% Patients out of total were found to have co-infection in which 29.5% patients have liver infection (14.2% HBs and 15.4% were HCV positive). Liver abnormalities in COVID-19 patients might be due to the viral infection in hepatocytes or due to the drug toxicity and systematic inflammation [14]. Liver injuries and morbidities is more common in severe COVID patients than patients that have mild condition with the disease [12]. However, other cause of liver morbidities such as non-alcoholic fatty liver disease, autoimmune hepatitis, alcoholic intolerance, liver disease related to consumption of alcohol, and their effect on prognosis of COVID-19 requires further justification and evaluation. Transplantation of liver also involves the risk of viral transmission from donor to recipient, this cause is already identified in the previous outbreak of SARS therefore donor testing and screening is must [15]. Our results also showed that 17.9% patients have kidney morbidities, 20.5% were heart patients and 32% were diabetic patients. Similar results were observed by [16-18]. The highest 14.1% comorbidities were found in the age range 51-60. The reason is same elder people are more susceptible to diseases or it might be due to the high number patients in this age group. Hematologic changes are associated with every disease Leukocytosis were found in 97% patients out of 78 comorbid patients, while in only 2.6% non-comorbid patients have leukocytosis. Leukopenia was detected in 61% non-morbid patients out of 200 COVID-19 patients. Thrombocytopenia was detected in 59% COVID-19 patients. Similar results were reported by [4,10]. The commodities and other associated risk must be managed as they worsen the COVID conditions and also affect the prognosis of COVID-19.
Conclusion
Fever, sore throat, dry cough and shortness of breath are more prevalent symptoms of COVID-19. The patients with previous cardiovascular diseases and other comorbid conditions may face greater risk of developing the disease into severe form. The comorbidities also affect the prognosis of COVID-19. 51-60 years of individuals are at high risk of getting infection. The hematologic changes are associated with COVID-19 which includes thrombocytopenia, leukocytosis, and leukocytopenia. Moreover, there is also a significant drop in number of WBC in COVID-19 infection. Serious steps to be taken regarding public health management and security to prevent from SARS-CoV-2 infection.
Acknowledgement
We are grateful to Hayatabad Medical Complex Peshawar, and Department of genetics hazara university Kp, Pakistan for providing research facilities.
Funding source
None.
Conflict of Interest
No conflict of interest.
Ethical Approval
Ethical approval was approved from Ethical and Research Committee of Hazara University Pakistan.
References
- Baud D, Qi X, Nielsen-Saines K, et al. Real estimates of mortality following COVID-19 infection. Lancet Infect Dis 2020; 20:773.
- Donnelly CA, Ghani AC, Leung GM, et al. Epidemiological determinants of spread of causal agent of severe acute respiratory syndrome in Hong Kong. Lancet 2003; 361:1761-1766.
- Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection?. Lancet Respir Med 2020; 8:e21.
- Guan W, Ni Z , Hu Y , et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708-1720.
- Huang C ,Wang Y ,Li X et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020; 395: 497-506.
- Kumar D, Tellier R, Draker R, et al. Severe Acute Respiratory Syndrome (SARS) in a liver transplant recipient and guidelines for donor SARS screening. Am J Transplant 2003; 3:977-981.
- Li B, Yang J, Zhao F, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol 2020; 109:1-8.
- Li LQ, Huang T, Wang YQ, et al. COVID‐19 patients' clinical characteristics, discharge rate, and fatality rate of meta‐analysis. J Med Virol 2020; 92:577-583.
- Li YX, Wu W, Yang T, et al. Characteristics of peripheral blood leukocyte differential counts in patients with COVID-19. Zhonghuaneike Za Zhi 2020; 59:e003.
- Misra DP, Agarwal V, Gasparyan AY, et al. Rheumatologists’ perspective on coronavirus disease 19 (COVID-19) and potential therapeutic targets. Clin Rheumatol 2020; 39:2055-2062.
- Qiu H, Wu J, Hong L, et al. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis 2020; 20:689-696.
- Cauchemez S, Fraser C, Van Kerkhove MD, et al. Middle East respiratory syndrome coronavirus: Quantification of the extent of the epidemic, surveillance biases, and transmissibility. Lancet infect Dis 2014; 14:50-56.
- Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: A systematic review and meta-analysis. Int J Infect Dis 2020; 94:91-95.
- Yeo C, Kaushal S, Yeo D. Enteric involvement of coronaviruses: is faecal–oral transmission of SARS-CoV-2 possible?. Lancet Gastroenterol Hepatol 2020; 5:335-337.
- Shi Z, Hu Z. A review of studies on animal reservoirs of the SARS coronavirus. Virus Res 2008; 133:74-87.
- Zhang C, Shi L, Wang FS. Liver injury in COVID-19: Management and challenges. Lancet Gastroenterol Hepatol 2020; 5:428-430.
- Zhang JJ, Dong X, Cao YY. Clinical characteristics of 140 patients infected by SARS-CoV-2 in Wuhan, China. Allergy 2020; 75:1730-1741.
- Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Ihteshamul Haq1, Shah Faisal2*, Abdullah3, Muhammad Romman4, Naumana Rhman5, Misbah Uddin6, Ghazala Zarin Afridi7, Sajjad Ali Shah2, Faheem Anwar8, Fazli Zahir9, Fatima Syed10, Naveed Iqbal9 and Shahzad Shoukat11
1Graduate School of Biotechnology and Oriental Medicine (Lab of Hangban Bio), Kyunghee University, Global Campus Suwon, South Korea2Department of Biotechnology, Bacha Khan University, Charsadda, KPK, Pakistan
3Department of Microbiology, Abdul Wali Khan University Mardan, KPK, Pakistan
4Department of Botany, University of Chitral KP, Pakistan
5Department of Pathology, Khyber Medical College, Peshawar KP, Pakistan
6Department of Biotechnology, Quaid Azam University, Islamabad, Pakistan
7Department Of Pathology, Khyber Girls medical College, Hayatabad Peshawar KP, Pakistan
8Department of Genetics, Hazara University, Mansehra KP, Pakistan
9Department of Allied Health Science, Iqra National University, Peshawar KP, Pakistan
10Institute of Biotechnology and Genetic Enginering, Agriculture University, Peshawar KP, Pakistan
11Department of Immunology, Postgraduate School Univeritas Airlangga Surbaya 60285, Indonesia
Citation: Ihteshamul Haq, Shah Faisal, Abdullah, Muhammad Romman, Naumana Rhman, Misbah Uddin, Ghazala Zarin Afridi, Sajjad Ali Shah, Faheem Anwar, Fazli Zahir, Fatima Syed, Naveed Iqbal, Shahzad Shoukat, Clinical Features and associated Comorbidities in 200 Cases of COVID-19 in Peshawar, Pakistan, J Res Med Dent Sci, 2023, 11 (1):11-16.
Received: 06-Dec-2022, Manuscript No. jrmds-22-84668; , Pre QC No. jrmds-22-84668(PQ); Editor assigned: 27-Dec-2022, Pre QC No. jrmds-22-84668(PQ); Reviewed: 10-Jan-2023, QC No. jrmds-22-84668(Q); Revised: 16-Jan-2023, Manuscript No. jrmds-22-84668(R; Published: 23-Jan-2023
