Research - (2020) Volume 8, Issue 1
Clinical and Histomorphomertic Evaluation of Effects of Platelet-poor Plasma and Platelet-rich Plasma on Healing of Extraction Sockets with Buccal Dehiscence: An Experimental Study in Dogs
Alaa Abbas Mahdi1*, Sahar Shakir Al-Adili1 and Zahid Ismael Mohammed2
*Correspondence: Alaa Abbas Mahdi, Department of Oral and Maxillofacial Surgery, Collage of Dentistry, University of Babylon, Iraq, Email:
Abstract
Background: Considerable alveolar bone loss occurs follows tooth extraction, this bone resorption can compromise dental implant procedure, for this reason, the concept of socket preservation techniques was introduced, in which different materials placed into the extraction socket immediately after extraction to minimize alveolar bone loss.
Aim of study: To evaluate the effects of autologous platelets poor plasma (PPP) and autologous platelets rich plasma (PRP) on the preservation of alveolar width in extraction socket with buccal dehiscence, and to evaluate the effect of PPP and PRP on the amount of new bone formation in extraction site after one and two months' duration.
Material and method: Six adult pointer dogs were selected for this study. The alveolar width was measured before extraction at a point 3 mm below the top of alveolar crest, after flap reflection 3 mm buccal dehiscence was created with trephine bur. Then the mandibular third premolar was extracted bilaterally. The extraction sites were randomly assigned to three groups: Platelets poor plasma, platelets rich plasma and control. The experiment was designed to permit the examination of the extraction site after one and two months. Then we measure the alveolar width reduction after one and two months and the amount of new bone formed in the extraction socket was measured histomorphometrically.
Results: There was no statistically significant difference in mean of alveolar width resorption between the three groups after one and two months, while the mean value of the amount of new bone formed in extraction socket was significantly higher in platelets rich plasma group after one month but not after two months. Platelets poor plasma has no statistically significant effects on bone formation in the extraction socket.
Conclusion: This study showed that platelets rich plasma increases the amount of new bone formation in the extraction socket after one month but it failed to have long term effects after two months. Platelets poor plasma has no statistically significant effect on the amount of new bone formation in the extraction socket after one and two months. Regarding the horizontal bone loss, it was not a statistically significant difference between all groups.
Keywords
Platelets rich plasma, Platelets poor plasma, Extraction site in dogs
Introduction
The alveolar process is a tooth dependent tissue and it develops when the teeth erupted. Furthermore, the shape and volume of the alveolar process are depending on the shape of the teeth, the long axis of the teeth, and its inclination [1]. After extraction of all teeth in elderly persons, the alveolar process will undergo atrophy [2-4]. The amount of this bone resorption was varied between individuals [4-6].
Many studies were found that marked horizontal and vertical alveolar bone resorption occurs following a tooth extraction, this alveolar bone resorption is more pronounced on the buccal than the lingual aspect of the alveolar ridge. This results in shifting the center of the alveolar ridge to the lingual side. Furthermore, this alveolar bone resorption was more pronounced during the early phase of healing [7-11]. Approximately two-third of horizontal bone resorption occurred in the first three months following tooth extraction. In this period, it was marked activity of osteoclast that result in resorption of the buccal and the lingual alveolar crest [8,9].
The causes for this bone loss are:
1. In preparation for removal of the Tooth and creation of buccal dehiscence, crevice incisions were made and full thickness flaps were elevated at both the buccal and lingual aspects of the alveolar process. It is well known that such surgical trauma that includes the separation of the periosteum and the rupture of its connective tissue attachment at the bone surface will induce an acute inflammatory response which in turn will mediate resorption of the surface layer of the alveolar bone in the exposed area [9].
2. The tooth is anchored to the jaws via the bundle bone into which the periodontal ligament fibers invest. Following the removal of a tooth, the bundle bone will lose its function and disappear. Hence, the early resorption of the bundle bone may in part explain the marked reduction of the height of the buccal wall that occurred between week 1 and week 4 of healing [9,12].
Bone resorption that follows tooth extraction makes insertion of dental implant difficult and has a negative effect on long term success of the dental implant and bad esthetic result of the following prosthesis. For these reasons, the socket preservation concept was introduced to reduce alveolar bone resorption in which different materials placed into the extraction socket immediately after tooth extraction to minimize bone resorption after tooth extraction and preserve the alveolar bone [13].
Marx et al. first introduced the technique of autologous platelet concentration to create the first platelet-rich plasma (PRP) for application in dental surgery [14]. PRP is a high concentration of autologous platelets within a small quantity of autologous plasma [15]. Marx et al. [14] reported that PRP promotes new bone formation and more maturation of the autologous bone graft in mandibular continuity defect.
PRP has a high number of platelets and it is an autologous source of growth factors such as transforming growth factor-β (TGF-β), plateletderived growth factor, and vascular endothelial growth factor [13,15]. Many studies have suggested the effectiveness of PRP in enhancing bone regeneration in bone graft however; others have shown that PRP has no benefit on bone healing [14-21]. Regarding the socket preservation, some studies have suggested that PRP alone increase bone formation rate and decrease healing time after tooth extraction [22,23]. But Alissa et al. were reported that bone formation rate in PRP treated socket was similar to the non-PRP treated socket [24].
Platelet-poor plasma (PPP) is a plasma fraction that contains few platelets. Few studies have attempted to evaluate the effects of PPP on bone regeneration. Hatakeyama et al. reported that platelets poor plasma preserve the width of alveolus more than platelets rich plasma (PRP) and platelets rich fibrin (PRF) also found that the amount of new bone was higher in PPP group in extraction socket with buccal dehiscence in dogs [13].
Subject and Methods
The research protocol was approved by the ethical committee of the Baghdad university college of dentistry. this study was carried out as a randomized prospective experimental study. It was performed during the period from December 2018 until the end of August 2019. Six pointer dogs with age one year and 10-17 kg were selected in this study. The animals were placed in the animal houses for 10 days before surgery for adaptation on the new environment.
The study conducted under general anesthesia, by an intramuscular injection of ketamine hydrochloride (0.5 ml/kg) with xylazine (0.5 mg/kg). Dental infiltration anesthesia by 2% lidocaine with epinephrine 1: 100,000. Crevicular incisions made from the first premolar to fourth premolar and reflection of full-thickness mucoperiosteal flap on the buccal and lingual side then sectioned the third premolar into two parts, mesial and distal root for careful tooth extraction. Right and left mandibular third premolars were extracted using elevators and forceps resulting in four extraction sockets per animal. The study design permits examination at one and two moths by extraction in one side and after one-month extraction the other side and sacrifice after completion of two months. The 24 sockets divided randomly into three groups.
Group A: Fill the socket with PPP.
Group B: Fill the extraction socket with PRP. Group C: Only suturing left as control then reposition the flap and suturing.
Before extraction the alveolar width was measured at point 3 mm below the top of the alveolar crest with Vernier caliper then buccal dehiscence was created with 3 mm diameter trephine bur. Then reposition the flap and suturing of extraction site with 3/0 silk suture with interrupted suturing. After two months all animals sacrificed with overdose of ketamine. Then the specimen contains extraction sites fixed with 10% formalin, for preparation of slides for histological examination.
Preparation of PPP and PRP
After general anesthesia. whole blood (10 ml) was collected from the jugular vein into sterile syringes then add to a tube containing 1 ml of the anticoagulant sodium citrate (0.9%). The whole blood first centrifuged at 2700 RPM for 10 min, and 2 ml of the top layer was collected as PPP. then, another sample of whole blood (10 ml) was added to another tube containing 1 ml of sodium citrate was collected by the same method used for the first sample. This sample was centrifuged at 2400 RPM for 8 min, and the top layers, with the buffy coat, was transferred to a new tube for further centerfire to remove the lowest layer that contained most of the red blood cells (RBCs). After further centrifugation of the transferred layers at 3800 RPM for 8 min, 2 ml of PRP was collected from the bottom layer, which is PRP. This PPP and PRP ware activated with 0.5 ml of 2% calcium chloride to obtain PPP and PRP gel [13].
Surgical procedure
After induction of general anesthesia, local anesthesia was infiltrated to the surgical site, incision and reflection of envelop mucoperiosteal flap from forth premolar to first premolar (Figure 1). Next separation between two roots of the third premolar for atraumatic extraction (Figure 2). Then with 3 mm trephine bur remove 3 mm from buccal crest for both mesial and distal roots to create 3 mm buccal dehiscence (Figure 3). Then extraction of mesial and distal roots as separated tooth and placing ether PPP or PRP gel or left as control (Figure 4).
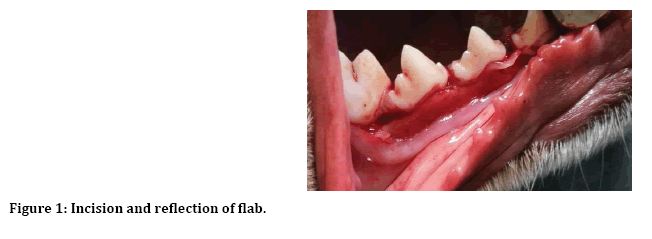
Figure 1: Incision and reflection of flab.
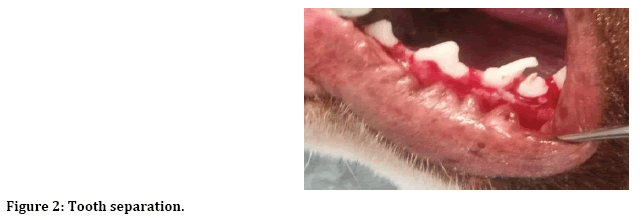
Figure 2: Tooth separation.
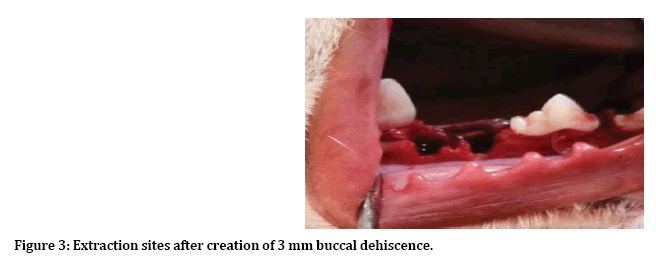
Figure 3: Extraction sites after creation of 3 mm buccal dehiscence.
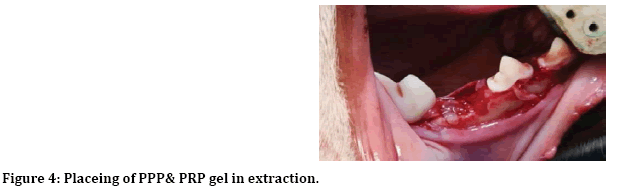
Figure 4: Placeing of PPP& PRP gel in extraction.
Sacrifice the animal and obtaining the samples After two months the doges were sacrificed with an overdose of ketamine and cutting the extraction site with a low-speed handpiece using surgical Disks and mandrel with normal saline irrigation (Figure 5). Then the block containing extraction site was placed in 10% formalin for slide preparation (Figure 6).
Figure 5: Cutting the sample.

Figure 6: Bone sample containing the extraction site after cutting.
Method of slide preparation
Decalcified of the bone sample in 10% formic acid for about 7 days after decalcification the sample dehydrated in a graded concentration of ethanol. Then impeded in paraffin wax and it cut in microtome in 4 μm width and stained with H&E stain and examined under the light microscope.
Morphometric measurement
Morphometric measurements were performed with the application image j on the photo took for the slid. by measurement the percentage of area in extraction site occupied by new bone using point counting procedure a modification of the method described by Schroeder et al. [1]. A lattice comprising 100 light points was superimposed over the ‘‘experimental unit’’; the relative volumes occupied by woven bone was calculated and expressed in percentage.
Statistics
Statistical analysis was performed by IBM SPSS statistics 25 and with descriptive statistics and analysis of variance ANOVA (one-way ANOVA).
Results
Clinical evaluation of alveolar ridge reduction Descriptive analysis of horizontal alveolar width reduction at point 3 mm below the top of alveolar crest after one and two months are shown in Table 1. After one month the highest reduction was in the control group mean (1.31 mm) and the lowest reduction was in the PRP group mean (1 mm) however it was not statistically significant P-value >0.05 (Table 2).
| Time | Groups | N | Min. | Max. | Mean | SD< |
|---|---|---|---|---|---|---|
| After one month | Control | 8 | 1 | 2 | 1.31 | 0.46 |
| PPP | 8 | 0.5 | 1.5 | 1.13 | 0.44 | |
| PRP | 8 | 0.5 | 1.5 | 1 | 0.38 | |
| After two months | Control | 4 | 1.5 | 2.5 | 1.9 | 0.48 |
| PPP | 4 | 1 | 2.5 | 1.75 | 0.65 | |
| PRP | 4 | 1.5 | 2 | 1.63 | 0.25 |
Table 1: Descriptive analysis of alveolar width reduction in mm at point 3 mm below the top of alveolar crest after one and two months.
| SOV | SS | DF | MS | F-cal. | P-value< |
|---|---|---|---|---|---|
| Between Groups | 0.369 | 2 | 0.184 | 0.976 | 0.393* |
| Within Groups | 3.965 | 21 | 0.189 | - | - |
| Total | 4.333 | 23 | - | - | - |
*P-value not statistically significant
SOV: Source of Variation
DF: Degree of Freedom
SS: Sum of Square
MS: Mean of Square
Table 2: Equality of mean of horizontal alveolar width resorption at a point 3 mm below the top of alveolar crest by ANOVA for all three groups after one month.
After two months the highest reduction was in the control group mean (1.9 mm) and the lowest reduction was in the PRP group mean (1.63 mm) however it was not statistically significant table P-value >0.05 (Table 3).
| SOV | SS | DF | MS | F-cal. | Sig< |
|---|---|---|---|---|---|
| Between Groups | 0.042 | 2 | 0.021 | 0.086 | 0.919* |
| Within Groups | 2.187 | 9 | 0.243 | ||
| Total | 2.229 | 11 |
*P-value not statistically significant
SOV: Source of Variation
DF: Degree of Freedom
SS: Sum of Square
MS: Mean of Square
Table 3: Equality of mean of alveolar width resorption at a point 3 mm below the top of alveolar crest by ANOVA for all three groups after two months.
The area occupied by new bone and bone marrow in the extraction site
Descriptive statistics of the area that was occupied by woven bone in the extraction socket after one month are shown in Table 4. The mean of the area occupied by woven bone was higher in the PRP group (mean 53.44) and lowest in the control group (mean 44.49) and it was statistically significant (P-Value < 0.05) (Table 5).
| Groups | N | Tissue | Mean | SD | Min. | Max. |
|---|---|---|---|---|---|---|
| Control | 4 | Bone | 44.49 | 1.33 | 43.33 | 46.36 |
| Marrow | 55.51 | 1.33 | 53.65 | 56.67 | ||
| PPP | 4 | Bone | 48.71 | 5.77 | 40.9 | 53.64 |
| Marrow | 51.29 | 5.77 | 46.36 | 59.1 | ||
| PRP | 4 | Bone | 53.44 | 3.87 | 48.48 | 57.93 |
| marrow | 46.56 | 3.87 | 42.07 | 51.52 |
Table 4: Descriptive analysis of area occupied by bone and bone marrow in the extraction site after one month.
| SOV | SS | DF | MS | F-cal. | P-value< |
|---|---|---|---|---|---|
| Between Groups | 160.326 | 2 | 80.163 | 4.804 | 0.038** |
| Within Groups | 150.184 | 9 | 16.687 | ||
| Total | 310.51 | 11 |
**P-value not statistically significant
SOV: Source of Variation
DF: Degree of Freedom
Table 5: Equality of mean of area occupied by woven bone in the extraction socket filled by PPP, PRP and control by ANOVA for all three groups after one months.
Descriptive statistics of the area that was occupied by woven bone in the extraction socket after two months are shown in Table 6. The mean of the area occupied by woven bone was higher in the PRP group (mean 50.93) and lowest in the control group (mean 47.945). However, it was not statistically significant (P-Value >0.05) (Table 7).
| Groups | N | Tissue | Mean | SD | Min. | Max. |
|---|---|---|---|---|---|---|
| Control | 4 | Bone | 47.945 | 3.83 | 44.74 | 53.33 |
| Marrow | 52.055 | 3.83 | 46.67 | 55.27 | ||
| PPP | 4 | Bone | 49.07 | 4.9 | 43.34 | 55.13 |
| Marrow | 50.93 | 4.9 | 44.87 | 56.67 | ||
| PRP | 4 | Bone | 50.93 | 3.82 | 47.5 | 56.38 |
| Marrow | 49.07 | 3.82 | 43.62 | 52.5 |
Table 6: Descriptive analysis of area occupied by bone and bone marrow in the extraction site after two months.
| SOV | SS | DF | MS | F-cal. | P-value< |
|---|---|---|---|---|---|
| Between Groups | 18.206 | 2 | 9.103 | 0.512 | 0.616* |
| Within Groups | 159.898 | 9 | 17.766 | ||
| Total | 178.103 | 11 |
*P-value not statistically significant
SOV: Source of Variation
DF: Degree of Freedom
SS: Sum of Square
MS: Mean of Square
Table 7: Equality of mean of area occupied by woven bone in the extraction socket filled by PPP, PRP and control by ANOVA for all three groups after two months.
Discussion
We choose the dog to this study because ease of manipulation, relativity small size, acceptable coast, availability, ease of housing, tolerance to surgery, there is a considerable amount of literature that comparing canine and human bone [25] also it is bone composition is most similar to human bone [26] and there are many literatures investigate the healing of extraction socket and socket preservation in doge so we can compare our result with these literatures.
Researchers in oral and maxillofacial surgery always attempt to improve on existing bonegrafting techniques and provide a faster and denser bony regenerate. Growth factors were shown to accelerate both bone and soft tissue healing. Platelets contain many growth factors in their granules [27,28]. Platelets have a store of growth factors such as platelets derived growth factor PDGF, transforming growth factor β TGFβ and vascular endothelial growth factor VEGF these growth factors are released when platelets activated [27]. It was reported that TGF-β1 and TGF-β2 inhibit bone resorption, osteoclast formation, and osteoclast activity, and trigger rapid maturation of collagen in early wounds [29,30]. PDGF increases the population of wound healing cells and recruits other angiogenic growth factors to the wound site [30]. Therefore, we suggested that increase the concentration of platelets in the extraction site may lead to improved and faster healing. Implant dentistry is aimed at placing the implant in anatomically, esthetically and long term function restorative position. Healing of extraction socket is characterized by alveolar bone resorption. this resorption result in restorative and esthetic challenges, which reduce available bone volume for implant placement, a major reduction in the alveolar ridge occurs in the first year following a tooth extraction, and two-thirds of this bone loss occurs within the first three months [10]. Many authors were reported enhanced bone formation and maturation rates after application of PRP in combination with autogenous bone, [14,17], xenograft [31] or allograft [32]. However other authors have reported no benefit of PRP when applied in conjunction with the autogenous bone [16,33,34], or xenograft [17] so it is unclear whether PRP promotes bone healing when applied into the bone defect. After application of PRP alone into human extraction site Anitua [35] observed more rapid epithelialization after four days and more mature bone after ten to sixteen weeks. After the application of PRP alone in animal studies, Schlegel et al. [36] observed increase the osseointegration of dental implants after two and four weeks but not after eight weeks. Zechner et al. [37] observed histomorphometrically improved osseointegration in the PRP group compared to the non-PRP group after three and six weeks but not after twelve weeks. Aghaloo et al. [16] were reported that PRP did not enhance bone formation in the non-critical sized cranial defects in rabbit after 1,2,3 and 4 months by radiographic and histomorphometric evaluation.
Regarding PPP there are limited studies on the effectiveness of PPP on bone healing Tajima et al. [38] was reported that PPP with bone marrow stromal cells (MSCs) and β-tri calcium phosphate (β-TCP) scaffolds promoted bone formation to a greater extent than PRP. And Hatakeyama et al. [13] observe that PPP preserve the alveolar width more effectively than PRP and PRF.
We found marked horizontal Bone resorption occurs in all groups after one and two months, This finding agree with Araújo and Lindhe [9] that found marked dimensional alternation occurred during the first eight weeks following extraction of mandibular premolars in dog model also it agree with Fickl et al. [39] that study dimensional changes of the alveolar ridge contour after different socket preservation techniques in dog model and found horizontal bone resorption occur in all groups.\
After one month all extraction sockets were filled with a new bone this is agreed with Cardaropoli et al. [12] that found the newly formed bone was filled most parts of the extraction socket in the extraction site of the dog after four weeks. but it disagrees with Hatakeyama et al. [13] that found the socket filled with the new bone after one month occurs only in the PPP group.
In extraction sockets representing two month of healing, hard tissue bridge was formed separating the extraction socket from overlying mucosa and connective tissue this agrees with Cardaropoli et al. [12], Araújo and Lindhe [9], and Vignoletti et al. [40] but disagree with Hatakeyama et al. [13] that found hard tissue bridge formed only in PRP group after eight weeks.
Within the limitation of this study, we found that PRP enhances the healing process statistically significant only after one month and it failed to have long term effects. PRP significantly increase bone formation after one month this agree with Gawai and Sobhana [41] that found PRP enhanced the osteogenic response in initial bone healing at 1-month duration but there was no added benefit in late bone healing. Also agree with Thorwarth et al. [42] that found that PRP has the only effect in the early period without long term effects. Also agree with Gerard et al. [43] that found the volume of new bone was significantly higher in PRP graft sites as compared with the non-PRP grafted site But it not agree with Aghaloo et al. [16] that report PRP alone has no significant effect over control group after 1 month also disagree with Hatakeyama et al. (13) that found the amount of new bone formation was higher in PPP group after four and eight weeks.
While PPP has no significant effect on the amount of new bone formation in the extraction site after one month. this finding may be due to the PPP has a low amount of platelets and a low amount of growth factors that stimulate the osteocytes to produce new bone. This finding disagrees with Hatakeyama et al. [13] that found the amount of new bone formation was higher in the PPP group after four and eight weeks. And this is the only published study that evaluates the effects of PPP on bone healing.
After two months there is no statistically significant difference between PRP, PPP, and control group this agree with Aghaloo et al. [16] that found PRP alone showed a histomorphometric tendency toward less bone area at 1, 2, and 4 months; however, this was not statistically significant when compared with control sites and also agree with Gawai, et al. [41] that found PRP enhanced the osteogenic response in initial bone healing at 1-month duration but there was no added benefit in late bone healing. But disagree with Gerard et al. [43] that found the volume of new bone was significantly greater in PRP sites when compared with the non-PRP graft sites. Also disagree with Kutkut et al. [44] that found PRP showed greater vital bone volume at 3 months with a rapid enhancement of bone healing compared to PRPfree collagen resorbable graft.
Conclusion
1. Marked horizontal Bone resorption occurs in all groups after one and two months and the mean of this bone resorption was not statistically significant between all groups.
2. After one month all extraction sockets were filled with thin trabeculae of woven bone and it was continuous with the old bone of socket walls, no hard tissue bridge formed at the entrance of the socket and oral mucous membrane seals the socket entrance in all groups.
3. In extraction sockets representing two months of healing hard tissue bridge was formed separating the extraction socket from overlying mucosa and connective tissue occurs in all groups.
4. After one month the amount of new bone formation in the extraction site treated with PRP was significantly higher compared to control groups. However, after two months the amount of new bone in the extraction socket was not statistically significant this indicates that the effect of PRP occurred in the early phase of the healing of the extraction socket.
5. The amount of new bone formation in the extraction site treated with PPP was not statistically significant compared to the control group after one and two months.
References
- Schroeder HE. Development, structure, and function of periodontal tissues. The periodontium. Springer 1986; 23-323.
- Atwood DA. A cephalometric study of the clinical rest position of the mandible: Part II. The variability in the rate of bone loss following the removal of occlusal contacts. J Prosthet Dent 1957; 7:544-552.
- Hedegard B. Some observations on tissue changes with immediate maxillary dentures. Dent Practitioner 1962; 13:70-78.
- Tallgren A. The continuing reduction of the residual alveolar ridges in complete denture wearers: A mixed-longitudinal study covering 25 years. J Prosthet Dent 1972; 27:120-132.
- Atwood DA. Some clinical factors related to rate of resorption of residual ridges. J Prosthet Dent 1962; 12:441-450.
- Carlsson G, Persson G. Morphologic changes of the mandible after extraction and wearing of dentures. A longitudinal, clinical, and x-ray cephalometric study covering 5 years. Odontol Revy 1967; 18:27-54.
- Camargo PM, Lekovic V, Weinlaender M, et al. Influence of bioactive glass on changes in alveolar process dimensions after exodontia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2000; 90:581-586.
- Schropp L, Wenzel A, Kostopoulos L, et al. Bone healing and soft tissue contour changes following single-tooth extraction: A clinical and radiographic 12-month prospective study. Int J Periodontics Restorative Dent 2003; 23:313-323.
- Araújo MG, Lindhe J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J Clin Periodontol 2005; 32:212-218.
- Fickl S, Zuhr O, Wachtel H, et al. Tissue alterations after tooth extraction with and without surgical trauma: A volumetric study in the beagle dog. J Clin Periodontol 2008; 35:356-363.
- Min S, Liu Y, Tang J, et al. Alveolar ridge dimensional changes following ridge preservation procedure with novel devices: Part 1–CBCT linear analysis in non‐human primate model. Clin Oral Implant Res 2016; 27:97-105.
- Cardaropoli G, Araujo M, Lindhe J. Dynamics of bone tissue formation in tooth extraction sites: An experimental study in dogs. J Clin Periodontol 2003; 30:809-818.
- Hatakeyama I, Marukawa E, Takahashi Y, et al. Effects of platelet-poor plasma, platelet-rich plasma, and platelet-rich fibrin on healing of extraction sockets with buccal dehiscence in dogs. Tissue Eng Part A 2013; 20:874-882.
- Marx RE, Carlson ER, Eichstaedt RM, et al. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1998; 85:638-646.
- Marx RE. Platelet-rich plasma: Evidence to support its use. J Oral and Maxillofac Surg 2004; 62:489-496.
- Aghaloo TL, Moy PK, Freymiller EG. Investigation of platelet-rich plasma in rabbit cranial defects: A pilot study. J Oral and Maxillofac Surg 2002; 60:1176-1181.
- Wiltfang J, Kloss FR, Kessler P, et al. Effects of platelet‐rich plasma on bone healing in combination with autogenous bone and bone substitutes in critical‐size defects: An animal experiment. Clin Oral Implant Res 2004; 15:187-193.
- Hokugo A, Ozeki M, Kawakami O, et al. Augmented bone regeneration activity of platelet-rich plasma by biodegradable gelatin hydrogel. Tissue Engineering 2005; 11:1224-1233.
- Choi BH, Im CJ, Huh JY, et al. Effect of platelet-rich plasma on bone regeneration in autogenous bone graft. J Oral and Maxillofac Surg 2004; 33:56-59.
- Aghaloo TL, Moy PK, Freymiller EG. Evaluation of platelet‐rich plasma in combination with freeze‐dried bone in the rabbit cranium: A pilot study. Clin Oral Implant Res 2005; 16:250-257.
- Klongnoi B, Rupprecht S, Kessler P, et al. Influence of platelet‐rich plasma on a bioglass and autogenous bone in sinus augmentation: An explorative study. Clin Oral Implant Res 2006; 17:312-320.
- Sammartino G, Tia M, Marenzi G di, et al. Use of autologous plateletrich plasma (PRP) in periodontal defect treatment after extraction of impacted mandibular third molars. J Oral Maxillofac Surg 2005; 63:766-770.
- Célio-Mariano R, de Melo WM, Carneiro-Avelino C. Comparative radiographic evaluation of alveolar bone healing associated with autologous platelet-rich plasma after impacted mandibular third molar surgery. J Oral Maxillofac Surg 2012; 70:19-24.
- Alissa R, Esposito M, Horner K, et al. The influence of platelet-rich plasma on the healing of extraction sockets: An explorative randomised clinical trial. Eur J Oral Implantol 2010; 3:121-134.
- Pearce A, Richards R, Milz S, et al. Animal models for implant biomaterial research in bone: a review. Eur Cell Mate. 2007; 13:1-10.
- Aerssens J, Boonen S, Lowet G, et al. Interspecies differences in bone composition, density, and quality: potential implications for in vivo bone research. Endocrinol 1998; 139:663-670.
- Banks R, Forbes M, Kinsey S, et al. Release of the angiogenic cytokine vascular endothelial growth factor (VEGF) from platelets: Significance for VEGF measurements and cancer biology. Br J Cancer 1998; 77:956.
- Maloney JP, Silliman CC, Ambruso DR, et al. In vitro release of vascular endothelial growth factor during platelet aggregation. Am J Physiol Heart Circulatory Physiol 1998; 275:1054-1061.
- Bonewald L, Mundy G. Role of transforming growth factor-beta in bone remodeling. Clin Orthop Related Res 1990; 250:261-276.
- Steenfos HH. Growth factors and wound healing. Scandinavian J Plastic Reconstruct Surg Hand Surg 1994; 28:95-105.
- Aghaloo TL, Moy PK, Freymiller EG. Evaluation of platelet-rich plasma in combination with anorganic bovine bone in the rabbit cranium: a pilot study. Int J Oral Maxillofac Implants 2004; 19:59-65.
- Kassolis JD, Rosen PS, Reynolds MA. Alveolar ridge and sinus augmentation utilizing platelet‐rich plasma in combination with freeze‐dried bone allograft: Case series. J Periodontol 2000; 71:1654-1661.
- Gerard D, Carlson ER, Gotcher JE, et al. Effects of platelet-rich plasma at the cellular level on healing of autologous bone-grafted mandibular defects in dogs. J Oral Maxillofac Surg 2007; 65:721-727.
- Mooren R, Merkx M, Bronkhorst E, et al. The effect of platelet-rich plasma on early and late bone healing: an experimental study in goats. Int J Oral Maxillofac Surg 2007; 36:626-631.
- Anitua E. Plasma rich in growth factors: preliminary results of use in the preparation of future sites for implants. Int J Oral Maxillofac Implants 1999; 14:529-535.
- Schlegel KA, Kloss FR, Kessler P, et al. Bone conditioning to enhance implant osseointegration: an experimental study in pigs. Int J Oral Maxillofac Implants 2003; 18:505-511.
- Zechner W, Tangl S, Tepper G, et al. Influence of platelet-rich plasma on osseous healing of dental implants: A histologic and histomorphometric study in minipigs. Int J Oral Maxillofac Implants 2003; 18:15-22.
- Tajima N, Sotome S, Marukawa E, et al. A three-dimensional cell-loading system using autologous plasma loaded into a porous β-tricalcium-phosphate block promotes bone formation at extraskeletal sites in rats. Materials Sci Eng 2007; 27:625-632.
- Fickl S, Zuhr O, Wachtel H, et al. Dimensional changes of the alveolar ridge contour after different socket preservation techniques. J Clin Periodontol 2008; 35:906-913.
- Vignoletti F, Discepoli N, Müller A, et al. Bone modelling at fresh extraction sockets: immediate implant placement versus spontaneous healing. An experimental study in the beagle dog. J Clin Periodontol 2012; 39:91-97.
- Gawai KT, Sobhana C. Clinical evaluation of use of platelet rich plasma in bone healing. J Maxillofac Oral Surg 2015; 14:67-80.
- Thorwarth M, Wehrhan F, Schultze-Mosgau S, et al. PRP modulates expression of bone matrix proteins in vivo without long-term effects on bone formation. Bone 2006; 38:30-40.
- Gerard D, Carlson ER, Gotcher JE, et al. Effects of platelet-rich plasma on the healing of autologous bone grafted mandibular defects in dogs. J Oral Maxillofac Surg 2006; 64:443-451.
- Kutkut A, Andreana S, Kim Hl, et al. Extraction socket preservation graft before implant placement with calcium sulfate hemihydrate and platelet‐rich plasma: A clinical and histomorphometric study in humans. J Periodontol 2012; 83:401-409.
Author Info
Alaa Abbas Mahdi1*, Sahar Shakir Al-Adili1 and Zahid Ismael Mohammed2
1Department of Oral and Maxillofacial Surgery, Collage of Dentistry, University of Babylon, Iraq2College of Veterinary Medicine, Iraq
Citation: Alaa Abbas Mahdi, Sahar Shakir Al-Adili, Zahid Ismael Mohammed, Clinical and Histomorphomertic Evaluation of Effects of Platelet-poor Plasma and Platelet-rich Plasma on Healing of Extraction Sockets with Buccal Dehiscence (An Experimental Study in Dogs), J Res Med Dent Sci, 2020, 8(1): 01-09.
Received: 04-Dec-2019 Accepted: 27-Dec-2019
