Review Article - (2023) Volume 11, Issue 1
Assessment the Cytotoxic Effect and Phytochemical Constituents of Ethyl Acetate Fraction of Iraqi Cassia glauca on Esophagus Cancer Cells
Shamam Kanaan M Abdlkareem* and Enas Jawad Kadhim
*Correspondence: Shamam Kanaan M Abdlkareem, Department of Pharmacognosy and Medicinal Plants, College of Pharmacy, University of Baghdad, Iraq, Email:
Abstract
Background: Cell lines obtained from cancer cells are usually used in experiments, such as use as a representative to cancer and to come up with a new treatment. The aim of the study: To explore the chemical constituents and assessment the anticancer activity against esophageal adenocarcinoma cell line of Cassia glauca (steams, leaves, flowers) parts where no phytochemical study had been done in Iraq on this plant.
Method: (Steams, leaves, flowers) parts of C. glauca defatted by maceration in n-hexane. The defatted plant parts were extracted by cold method, the aqueous ethanol 85% used as a solvent for extraction and the extract fractionated by chloroform, ethyl acetate and n-butanol. The ethyl acetate fraction then analyzed by High Performance Liquid Chromatography (HPLC) to investigate its constituents. The MTT assay used to evaluate the anticancer activity by employment 96 well plates, the cell lines implanted by (1 × 104) cells/well. And 24 h later different concentration had been used to treat the SK-GT4 cell lines. By removing the medium after 72 h of treatment the cell viability was measured, 28 μL of MTT solution of concentration (2 mg/mL) were added and then the cells were incubated for 1.5 h at 37°C. When the MMT solution where removed any crystals sticked on the walls by addition of 130 μL of DMSO (Dimethyl Sulphoxide) were solubilized, then another incubation at 37°C with shaking for 15 min. On a micro plate reader at 492 nm (test wavelength) the absorbance where determined.
Conclusion: Plant extract from C. glauca ethyl acetate fraction showed very acceptable anticancer activity because it inhibits tumor progression particularly in human esophagus adenocarcinoma cell line. Our results encourage that this plant extract fraction is hopeful as anticancer reagent.
Keywords
Cassia glauca, Anti-cancer activity, SK-GT-4, HPLC, Phytochemical
Introduction
In 2018, the number of esophageal cancer worldwide estimated as 572000 new cases which include 85000 (15%) cases of Esophageal Adenocarcinoma (EAC) [1]. And the burden from EAC is going to increase specially in the high income countries, chiefly among men and by 2030, particularly in the United Kingdom and the Netherlands 1 in 100 are probable to have EAC during their lifespan [2]. And the esophageal cancer is known by its aggressiveness, due to the absence of serosa in the esophageal wall which is a serious factor in spreading of the cancer and makes it pervasive by many pathways including direct extension, hematogenous metastasis and lymphatic spread. Concerning the prognosis as no anatomical barrier, the tumor diffuse readily into the vicinal organs of the thorax and neck which consist of the trachea, larynx, pericardium, thyroid gland, diaphragm and lung [3]. Thus, an important demand to evolve new, affordable and effective drugs [4]. An extracts from a number of herbal plants had been proved to have anticancer activities both in vitro and in vivo by several researches [5,6]. Due to enhancement the immune system as improved by many researches [7]. So the natural and herbal products have been rated as precious source for treatment of diseases and malignant and about 60% of drugs at present are used as a cancer medication it is source is the natural products [8,9].
The cassia is one of four large genus of the family leguminosaea (worldwide distribution family and had estimated to 16000 to 19000 species) and amongst the twenty five largest genera of dicotyledonous plants [10,11].
C. glauca is a tall tree, with glabrous branchets. Leaves distinctly petioled, 1/2-3/4 ft; leaflets ovate, acute or sub-obtuse, 2-4 in, flowers large, bright yellow. Pod flat; thin strap shaped 6-8 in. Long and 1/2-3/4 in broad, distinctly stalked, 20-30 seeded [12]. It has diverse common names as C. surattensis, C. sulfurea and Senna arborescens. It is native to East Indies, distributed from the Himalayas, in India through Ceylon and the Polynesian island to Australia [13]. Cultivated in the tropical countries in Asia such as Sri Lanka, Thailand, Burma and Malaysia [14]. And is known to have escaped into the wild, naturalizing in many of places (Irwin and Barneby, 1982). In the ayurvedic system the C. glauca total aerial parts used as depressant to the central nervous system, antimalarial, purgative and diuretic [15]. Leaves are used for blennorrhagia and also, in folk medicine the bark and leaves are usually utilized for the treatment of gonorrhea and diabetes (Indian medicinal plants Kirtikar and Basu, 1944). Whilst in indigenous system of medicine the seeds oil are used in the treatment of leucoderma and skin diseases [16]. In traditional Brazilian medicine it has been used for the treatment of flu, cold, fever and headache, while the decoction of the roots is commonly used to treat snake bites [17,18]. Many reports have shown the cassia species possessing anti-diabetic, anti-microbial, antimalarial, anti-cancer and hepatoprotective [19,20]. Finally, some compounds had been isolated from C. glauca including steroids (beta sitosterol), anthraquinones (emodin, chrysophanol and physcion) and flavonoids (Apigenin, Kaempferol) [20-22].
So, investigation to esophagus anticancer activity and isolation, Identification and characterization of some pharmacological active compounds of C. glauca ethyl acetate fraction cultivated in Iraq will be done.
Materials and Methods
Plant material collection
The (steams, leaves, flowers) parts of C. glauca were taken from side road of Baghdad city in April 2021. The plant was specified and authenticated by Prof Dr. S Abass department of biology college of sciences university of Baghdad. The parts were washed, dried at room temperature and crushed by using a mechanical grinder to powder.
Method of work
The dried powder is weighted and defatted by n-hexane solvent to exclude the chlorophyll and waxy materials, the defatted powder is extracted by maceration with 85% ethanol for 7 days then the extract dried by rotary evaporator and weighted after that dissolved in water and successively fractionated by using separator funnel with chloroform, ethyl acetate and n-butanol solvents [23]. Each fraction is dried by rotary evaporator and weighted for further analysis.
Preliminary qualitative and phytochemical analysis
Chemical tests done by using the ethyl acetate extract of the plant and standard procedures carried out to identify the active constituents (Table 1) [24,25].
| Constituent | Test |
|---|---|
| Flavonoids | Alkaline test: 1 ml from ethyl acetate extract and few drops of sodium hydroxide solution. A yellowish precipitate indicating a positive test for flavonoids. Shinoda's test: 1 ml from ethyl acetate extract treated with magnesium and concentrated HCL. Red orange color indicate the presence of flavonoids. |
| Tannin and phenolic compounds | 5% ferric chloride: 1 ml of ethyl acetate extract mixed with 1 ml of FeCl3 solution (5% w/v) to produce dark green or blue black precipitate indicates the presence of tannins. 10% lead acetate: 1 ml of ethyl acetate extract mixed with 1 ml of 10% lead acetate solution a white precipitate indicates the presence of tannins. |
| Alkaloids | Dragendroff’s test: A small portion of ethyl acetate fraction was treated with 1% HCl on steam bath then Dragendroff’s reagent (solution of potassium bismuth iodide) was added. Orange brown precipitation due to double salt formation indicates the presence of nitrogenous substances. Mayer’s reagent: A small portion of ethyl acetate fraction was treated with 1% HCl on steam bath then Mayer’s reagent (1.35 gm mercuric chloride in 60 ml water +5 gm potassium iodide in 10 ml water) was added, creamy precipitate due to double salt formation indicates the presence of alkaloids. |
| Steroids | Libermann burchard test: A small portion of ethyl acetate fraction was dissolved in 5 ml of chloroform, then the chloroform layer was dried over anhydrous sodium sulfate and later on mixed with 10 drops of acetic anhydride and 2 drops of concentrated sulphuric acid are then added, bluish green color indicates the presence of steroidal nucleus as oxidation occur in steps |
| Anthraquinones | Brontrager’s test: A small portion of ethyl acetate fraction was shacked with 3 ml of benzene and then filtered, mixed with 3 ml of 10% ammonium hydroxide solution, pink color in the ammonical phase indicate the presence of anthraquinones |
| Cardiac glycoside | Keller-kiallian’s test: A small portion of ethyl acetate fraction was mixed with 2 ml of glacial acetic acid and 1 drop of 0.1% ferric chloride solution then add 1 ml of H2SO4 (drop by drop) were add, formation of green blue color indicates the presence of cardiac glycoside. |
Table 1: Chemical tests used to identify the active constituents in ethyl acetate fraction.
High Performance Thin Layer Chromatography (HPLC)
Standard and sample solutions (rutin, isorhmentine, quercetin and ethyl acetate F.) were dissolved in absolute methanol.
HPLC technique Waters 1525 was used to check the constituents found in the ethyl acetate fraction. The mobile phase composed of 1% aqueous acetic acid solution (B) and 100% MeOH (C). The samples elution done by this gradient: 90% B and 10% C from 0 to 6 min, 84% B and 16% C from 7 to 25 min, 72% B and 28% C from 26 to 37 min, 65% B and 35% C from 38 to 47 min, 50% B and 50% C from 48 to 64 min and 90% B and 10% C from 65 to 70 min, to restore the initial conditions so a second sample can be injected. 0.8 mL/min the flow rate was, 5 μL was the injection volume. Hypersil gold column (150 × 4.6) at 25°C temperature. The detection of flavonoids was done by UV detector at λ=278 nm. The identification based on retention time for the compound by comparing with standards in the same conditions [26].
Chemicals and reagents
The chemicals, reagents and instruments used listed in the below Table 2.
| No. | Item | Company | Country |
|---|---|---|---|
| 1 | Trypsin/EDTA | Capricorn | Germany |
| 2 | DMSO | Santacruz biotechnology | USA |
| 3 | RPMI 1640 | Capricorn | Germany |
| 4 | MTT stain | Bio world | USA |
| 5 | Fetal bovine serum | Capricorn | Germany |
| 6 | incubator | Cypress diagnostics | Belgium |
| 7 | Micro titer reader | Gennex lab | USA |
| 8 | Laminar flow hood | K and K scientific supplier | Korea |
| 9 | Micropipette | Cypress diagnostics | Belgium |
| 10 | Cell culture plates | Santa Cruz biotechnology | USA |
Table 2: Chemicals, reagents and instruments used.
Maintenance of cell cultures
SK-GT-4 cell line was preserved in MEM solution (Minimal Essential Medium) added 10% fetal bovine, 100 μg/mL streptomycin and 100 units/mL penicillin. Cells were passaged using trypsin EDTA incubated at 37°C and reseeded at 50% confluence twice a week [27].
Cytotoxicity assay
To define the cytotoxic activity, the MTT assay used which was done by 96 well plates, the cell lines implanted with (1 × 104) cells/well [28]. And after 24 h different concentration had been used to treat the SK-GT4 cell lines. By removing the medium after 72 h of treatment the cell viability was measured, 28 μL of MTT solution of concentration (2 mg/mL) were added and then the cells were incubated for 1.5 h at 37°C. When the MMT solution where removed any crystals sticked on the walls by addition of 130 μL of DMSO (Dimethyl Sulphoxide) were solubilized, then another incubation at 37°C with shaking for 15 min [29]. On a micro plate reader at 492 nm (test wavelength) the absorbance where determined; in triplicates the assay done. The percentage of cytotoxicity to cell growth was calculated by this equation [30].
% Cell viability=(Absorbance of treated cell/Absorbance of non-treated cell) × 100
% Cytotoxicity=100-Cell viability
Statistical analysis
Using by usage of graph pad prism 6 and an unpaired T test the data were analyzed [31]. And by the mean ± SD of triplicate measurements the values were presented [32].
Results and Discussion
Phytochemical analysis
The results of phytochemical analysis are given in below Table 3.
| Tested component | Type of test | Result |
|---|---|---|
| Flavonoids | Alkaline | +ve |
| Shinoda's | +ve | |
| Tannin and phenolic compounds | 5% ferric chloride | +ve |
| 10% lead acetate | +ve | |
| Alkaloids | Dragendroff’s | +ve |
| Mayer’s reagent | -ve | |
| Steroids | Libermann-Burchard | -ve |
| Anthraquinones | Brontrager’s | -ve |
| Cardiac glycosides | Keller-Kiallian’s | -ve |
| (+,-) represent presence and absence of phytoconstituents respectively. | ||
Table 3: Qualitative phytochemical analysis of C. glauca (steams, leaves, flowers) ethyl acetate fraction.
The results of preliminary phytochemical screening of plant ethyl acetate fraction showed the presence of, flavonoids, tannins alkaloids and steroids in the (steams, leaves, flowers) parts of Iraqi species of this plant and the absence of anthraquinones and cardiac glycosides in the same plant parts.
The identification of compounds by HPLC done by comparing the obtained retention times for both standards and ethyl acetate fraction. Their chromatograms of standards (Figure 1) compared with ethyl acetate fraction chromatogram (Figure 2).
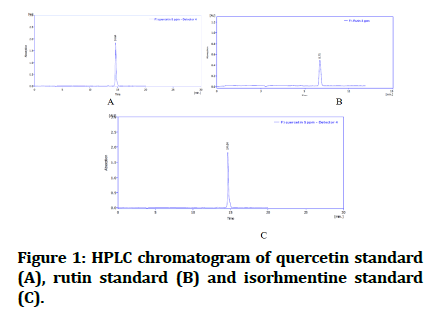
Figure 1: HPLC chromatogram of quercetin standard (A), rutin standard (B) and isorhmentine standard (C).
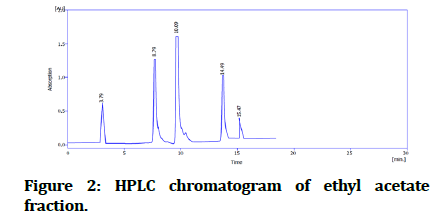
Figure 2:HPLC chromatogram of ethyl acetate fraction.
In this study, the cytotoxic effect of C. glauca ethyl acetate fraction against cancer cell was evaluated by using SK-GT4 cell line. The results demonstrate that a very noteworthy cytotoxic activity against the EAC cell lines as represent (Figure 3). Below, the data showed that there is ability in ethyl acetate fraction to curb the growth of esophagus cancer cell line and significantly, in concentration dependent manner (Figure 4).
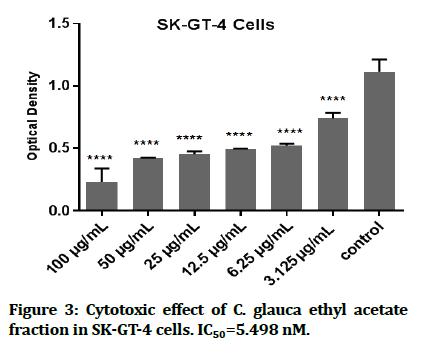
Figure 3:Cytotoxic effect of C. glauca ethyl acetate fraction in SK-GT-4 cells. IC50=5.498 nM.
Figure 4:Control untreated SK-GT-4 cells.
IC50=5.498 50 nm of the ethyl acetate fraction (which is the half inhibitory concentration usually use to measure of the potency of a drug to inhibit a biochemical or biological substance). It may be possible that the antioxidant activities of this fraction are likely due to the presence of flavonoids. The flavonoids can inhibit injury caused by free radicals by scavenging of reactive oxygen species, metal chelating activity, activation of antioxidant enzymes, tocopheryl radicals reduction, mitigation of oxidative stress caused by (nitric oxide, increase in uric acid) and inhibition of oxidases. Also antioxidant properties of low molecular antioxidants [33].
And in comparison of IC50 of C. glauca ethyl acetate fraction with previous study done on the same cell line and same MTT assay but, with different concentrations for paclitaxel (FDA approved drug for gastro esophageal adenocarcinoma) the IC50 was 50 nm which is higher concentration by about 10 times than C. glauca fraction IC50 concentration [34]. Another study done also on same cell line in the same condition for rumex acetosella methanol extract IC50=42.62 nm, so we found that our plant is much more active against EAC (Figure 5) [35].
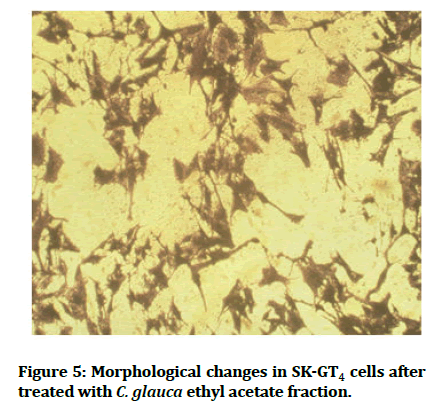
Figure 5:Morphological changes in SK-GT4 cells after treated with C. glauca ethyl acetate fraction.
In spite of many compounds isolated from this plant are being precisely studded for their anticancer activity, it is becoming obvious that the benefit effects of whole plant parts use is because of the synergistic effect of the compounds which present rather than the use of single constituent alone [36,37].
Conclusion
C. glauca ethyl acetate fraction extract of (steams, leaver, flower) may be has promising anticancer activity against EAC. The activity may related to the flavonoids exist in the fraction in addition to synergistic effect.
References
- ArnoldM, FerlayJ, Van Berge HenegouwenMI, et al. Global burden of esophageal and gastric cancer by histology and sub site in 2018. Gut 2020; 69:1564-1571. [Crossref][Googlescholar][Indexed]
- Arnold M, Laversanne M, Brown LM, et al. Predicting the future burden of esophageal cancer by histological subtype: International trends in incidence up to 2030. Am J Gastroenterol 2017; 112:1247-1255. [Crossref][Googlescholar][Indexed]
- Abbasi AM, Shah MH, Li T, et al. Ethno medicinal values, phenolic contents and antioxidant properties of wild culinary vegetables. J Ethnopharmacol 2015;162:333-345. [Crossref][Googlescholar][Indexed]
- Coseri S. Natural products and their analogues as efficient anticancer drugs. Mini Rev Med Chem 2009;9:560-571. [Crossref][Googlescholar][Indexed]
- Gill CI, Boyd A, McDermott E, et al. Potential anticancer effects of virgin olive oil phenolson colorectal carcinogenesis models in vitro. Int J Cancer 2005;117:1-7. [Crossref][Googlescholar][Indexed]
- Chiu LCM, Ho TS, Wong EYL, et al. Ethyl acetate extract of Patrinia scabiosaefolia downregulates anti apoptotic Bcl-2/Bcl-XL expression and induces apoptosis in human breast carcinoma MCF-7 cells independent of caspase-9 activation. J Ethnopharmacol 2006;105:263-268. [Crossref][Googlescholar][Indexed]
- Chen X, Hu ZP, Yang XX, et al. Monitoring of immune responses to a herbal immunomodulator in patients with advanced colorectal cancer. Int Immunopharmacol 2006; 6:499-508. [Crossref][Googlescholar][Indexed]
- Yin X, Zhou J, Jie C, et al. Anticancer activity and mechanism of Scutellaria barbata extract on human lung cancer cell line A549. Life sci 2004;75:2233-2244. [Crossref][Googlescholar][Indexed]
- Gordaliza M. Natural products as leads to anticancer drugs. Clin Transl Oncol 2007;9:767-776. [Crossref][Googlescholar][Indexed]
- Molares S, Ladio A. The usefulness of edible and medicinal fabaceae in argentine and chilean patagonia: Environmental availability and other sources of supply. Evid Based Complement Altern Med 2012. [Crossref][Googlescholar][Indexed]
- Hasanuzzaman M, Araujo S, Gill SS. The plant family fabaceae. Springer, Singapore, 2020. [Googlescholar][Indexed]
- Hooker JD. Flora of British India. Biodiversity Hertiage Library (BHL), London, 1879; 2. [Crossref][Googlescholar]
- Allen ON, Allen EK. The leguminosae, a source book of characteristics, uses and nodulation. Univ of Wisconsin Press, 1981. [Crossref][Googlescholar][Indexed]
- Veerapur VP, Pratap V, Thippeswamy BS, et al. Polyphenolic enriched extract of Cassia glauca Lamk, improves streptozotocin induced type 1 diabetes linked with partial insulin resistance in rats. J Ethnopharmacol 2017;198:489-498. [Crossref][Googlescholar][Indexed]
- Chopra RN, Nayar SL, Chopra IC. Glossary of Indian medicinal plants. Published by NAG publishers, New Delhi, India. 2002. [Googlescholar][Indexed]
- Singh RB. Nature of seed polysaccharide isolated from Cassia glauca Lam. plant. Nature. Int J Multidiscip Sci 2018; 1-2.
- Phuse SS, Khan ZH. Assessment of hemolytic effect of Cassia flower extracts on human RBCs. J Drug Deliv Ther 2018;8:18-20. [Crossref][Googlescholar][Indexed]
- Burkill IH. A dictionary of the economic products of the malay peninsula. A dictionary of the economic products of the malay peninsula, 2nd edition, 1966. [Googlescholar][Indexed]
- Kittur BS, Srinivas Y, Deshp SR. Evaluation of leaf and stem extracts from Cassia Glauca L. For antimicrobial activity. Int J Pure Appl Zool 2015; 3:908-102.
- El-Sayed MM, Abdel Aziz MM, Abdel Gawad MM, et al. Chemical constituents and cytotoxic activity of Cassia glauca lan leaves. Life Sci J 2013; 10:1617-1625. [Googlescholar][Indexed]
- Gervasi O, Murgante B, Lagana A, et al. Springer Science and Business Media, Computational Science and Its Applications-ICCSA 2008: International Conference, June 30-July 3, 2008 Proceedings, Perugia, Italy, 2008; 2:1142. [Crossref][Googlescholar][Indexed]
- Hafez S, Othman S, Ibrahim H, et al. Chemical constituents and biological activities of Cassia genus. Archives of Pharmaceutical Sciences Ain Shams University 2019; 3:195-227. [Crossref][Googlescholar][Indexed]
- Hussein AM, Kadum EJ. Identification and Isolation of caffeic, chlorogenic and ferulic acids in aerial parts of Capparis spinosa wildly grown in Iraq. Iraqi J Pharm Sci 2020; 29:185-193. [Crossref][Googlescholar][Indexed]
- Morsy N. Phytochemical analysis of biologically active constituents of medicinal plants. Main Group Chem 2014; 13:7-21. [Googlescholar]
- Sarker SD, Latif Z, Gray AI. Natural products isolation. Nat pro isolation 2006; 1:25. [Googlescholar][Indexed]
- Stoenescu AM, Trandafir I, Cosmulescu S. Determination of phenolic compounds using HPLC-UV method in wild fruit species. Horticulturae 2022;8:84. [Crossref][Googlescholar][Indexed]
- Al-Shammari AM, Alshami MA, Umran MA, et al. Establishment and characterization of a receptor negative, hormone non-responsive breast cancer cell line from an Iraqi patient. Breast Cancer 2015;7:223. [Crossref][Googlescholar][Indexed]
- Adil BH, Al-Shammari AM, Murbat HH. Breast cancer treatment using cold atmospheric plasma generated by the FE-DBD scheme. Clin Plasma Med 2020; 19:100103. [Crossref][Googlescholar][Indexed]
- Abdullah SA, Al-Shammari AM, Lateef SA. Attenuated measles vaccine strain have potent oncolytic activity against Iraqi patient derived breast cancer cell line. Saudi J Biol Sci 2020;27:865-872. [Crossref][Googlescholar][Indexed]
- Al-Shammari AM, Jalill RDA, Hussein MF. Combined therapy of oncolytic Newcastle disease virus and rhizomes extract of Rheum ribes enhances cancer virotherapy in vitro and in vivo. Mol Biol Rep 2020;47:1691-1702. [Crossref][Googlescholar][Indexed]
- Mohammed MS, Al-Taee MF, Al-Shammari AM. Caspase dependent and independent anti-hematological malignancy activity of AMHA1 attenuated new castle disease virus. Int J Mol Cell Med 2019;8:211. [Crossref][Googlescholar][Indexed]
- Al-Ziaydi AG, Al-Shammari AM, Hamzah MI, et al. Newcastle disease virus suppress glycolysis pathway and induce breast cancer cells death. Virusdisease 2020;31:341-348. [Crossref][Googlescholar][Indexed]
- Sahib HB, Ismail Z, Othman NH, et al. Orthosiphon stamineus Benth methanolic extract enhances the anti-proliferative effects of tamoxifen on human hormone dependent breast cancer. Int J Pharmacol 2009;5:273-276. [Crossref][Googlescholar]
- Kim SJ, Chung MJ, Kim JS, et al. Deciphering the role of paclitaxel in the SKGT4 human esophageal adenocarcinoma cell line. Int J Oncol 2011; 39:1587-1591. [Crossref][Googlescholar][Indexed]
- Ahmed OH, Abdulhussein AJ, Kadhim EJ. The cytotoxic effect of iraqi rumex acetosella against breast and esophagus cancer cells. Indian J Forensic Med Toxicol 2022;16:929. [Crossref][Googlescholar][Indexed]
- Liu RH. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am J Clin Nutr 2003; 78:517S-520S. [Crossref][Googlescholar][Indexed]
- Karna P, Chagani S, Gundala SR, et al. Benefits of whole ginger extract in prostate cancer. Br J Nutr 2012; 107:473-484. [Crossref][Googlescholar][Indexed]
Author Info
Shamam Kanaan M Abdlkareem* and Enas Jawad Kadhim
Department of Pharmacognosy and Medicinal Plants, College of Pharmacy, University of Baghdad, IraqCitation: Shamam Kanaan M Abdlkareem, Enas Jawad Kadhim, Assessment the Cytotoxic Effect and Phytochemical Constituents of Ethyl Acetate fraction of Iraqi Cassia glauca on Esophagus Cancer Cells, J Res Med Dent Sci, 2023, 11 (01): 103-108.
Received: 28-Oct-2022, Manuscript No. JRMDS-22-65199; , Pre QC No. JRMDS-22-65199 (PQ); Editor assigned: 02-Nov-2022, Pre QC No. JRMDS-22-65199 (PQ); Reviewed: 16-Nov-2022, QC No. JRMDS-22-65199; Revised: 27-Dec-2022, Manuscript No. JRMDS-22-65199 (R); Published: 04-Jan-2023
