Research - (2020) Volume 8, Issue 3
Antibacterial and Antibiofilm Effects of Virgin Coconut and Virgin Olive Oils on Streptococcus sobrinus and Lactobacillus casei
Nur Hafizah Hazmi1, Zaleha Shafiei2, Ahmad Shuhud Irfani Zakaria1, S Nagarajan MP Sockalingam1 and Alida Mahyuddin1*
*Correspondence: Alida Mahyuddin, Department of Family Oral Health, Faculty of Dentistry, University Kebangsaan Malaysia, Malaysia, Email:
Abstract
Background: To determine the effect of virgin coconut and virgin olive oils on the growth of Streptococcus sobrinus and Lactobacillus casei using Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC), to observe the effects of these oils on the morphology of Streptococcus sobrinus and Lactobacillus casei, to investigate the ant adherence and antibiofilm effects of these oils towards Streptococcus sobrinus and Lactobacillus casei in single- and dual-species biofilms.
Materials and method: Broth microdilution technique was implemented to determine the MIC of the oils in a 96-wells microtiter plate, followed by MBC using the sub-cultured method. Chlorhexidine (0.12% CHX) and broth served as positive and negative controls respectively. All specimens that exhibited MIC were examined under a Transmission Electron Microscope. Both oils at MIC and subMIC were also tested for antiadherence and antibiofilm properties.
Results: Virgin coconut oil showed higher antibacterial activities against Streptococcus sobrinus and Lactobacillus casei compared to virgin olive oil but were not statistically significant (p=0.40 and p=0.34, respectively). Both oils were bactericidal at 100% concentration. Significant cytological changes in cell morphology were observed in both bacteria after exposure to both oils. Virgin olive oil showed higher antiadherence activity while virgin coconut oil exerted higher antibiofilm activity.
Conclusions: Virgin coconut and virgin olive oils are as effective as chlorhexidine as an alternative mouthwash. The effectiveness of virgin olive oil is at the initiation stage of plaque formation as it has superior antiadherence activity. However, once the plaque has formed, the use of virgin coconut oil as a mouthwash is advocated as it has more superior antibiofilm activity.
Keywords
Virgin coconut oil, Virgin olive oil, Minimum inhibitory concentration, Oral bacteria, Antibiofilm, Antiadherence
Abbrevations
VOO-Virgin Olive Oil, VCO-Virgin Coconut Oil, CHX-Chlorhexidine, S. sobrinus-Streptococcus sobrinus, L. casei- Lactobacillus casei
Introduction
In southeast Asia, the burden of early childhood caries (ECC) shows an increasing trend where caries prevalence in the 5 and 6-year-olds is ranged between 25% and 95% [1]. The treatment for ECC is extremely costly and causes a great burden on parents as well as the health care system [2]. Thus, it is important to implement preventive measures at an early stage as ECC is mainly preventable. In caries prevention, the use of mouthwash as an antiplaque agent can provide significant benefits to patients. This is particularly true in those who cannot maintain adequate plaque control, for example, in young children or physically disabled patients such as cerebral palsy. Thomas et al. reported reduced plaque biofilm following treatment with Chlorhexidine (CHX) mouthwash in severe ECC patients [3]. Despite being recognized as the gold standard for antiplaque agents, long term usage of CHX is not recommended because of various side effects such as brown discoloration, taste perturbation and enhanced supragingival plaque formation [4].
As most existing antimicrobial agents are synthetic based, a natural antimicrobial with fewer side effects would be most ideal for children and those with a disability. Furthermore, the emergence of resistant bacteria strains towards existing antimicrobial agents has resulted in the use of natural-based oil against oral microorganisms [5]. On social media, ongoing discussion indicates consumers’ preference for natural products especially those that can be easily obtained locally [6]. Currently, virgin coconut (VCO) and virgin olive oils (VOO) are two edible oils that are readily available in Malaysia. They are known food products and their potentials as alternative antimicrobial agents have been mentioned due to fewer reported adverse side effects, better patient tolerance, relatively inexpensive and biodegradability [7].
Most studies have evaluated the effects of these oils against Streptococcus mutans (S. mutans), which is the most cariogenic bacteria to colonise the oral environment [8]. However, cariogenic biofilm is composed of a multi-species microbial community, therefore, other Streptococci and Lactobacilli species are also implicated in the onset and progression of childhood caries. No study has been conducted to determine the effect of VCO and VOO against other oral microorganisms.
Hence, this study was set out to determine the antibacterial effects of VCO and VOO on Streptococcus sobrinus (S. sobrinus) and Lactobacillus casei (L. casei) and compare them with 0.12% CHX.
Materials and Methods
Ethical approvals from the relevant ethics committee were obtained before the commencement of the study (Reference: UKM PPI/111/8/JEP-2017-796).
Preparation of oils and bacterial suspension
The VCO was obtained from the Malaysian Agriculture Research and Development Institute (MARDI, Malaysia), whereas, VOO was purchased from a local supermarket, with commercial name Extra Virgin Olive Oil by BorgesTM (Spain). A total of 1 % of ethanol (HmbG, Germany) was used to increase the solubility of the 2 oils in the broths for both bacteria.
The 2 strains of bacteria selected were Streptococcus sobrinus Coykendall ATCC 33478TM (ATCC, USA) and Lactobacillus casei (Orla-Jensen) ATCC 393TM (ATCC, USA). The medium for each bacterium was Brain Heart Infusion (BHI) Agar/Broth and de Man, Rogosa and Sharpe (MRS) Agar/Broth, respectively.
For dual species, the standardized suspensions of the two bacteria were mixed (1:1 vol/vol). The standardized bacteria suspension was prepared to a dilution of 106 CFU/ml, for both S. sobrinus (OD590 nm of 0.026) and L. casei (OD590nm of 0.032) in their respective media.
Determination of minimum inhibitory and minimum bactericidal concentrations of oils
MIC is considered as the lowest concentration of oil that would result in the visible absence of bacterial growth. In this study, the MICs were determined on oils that showed antimicrobial activity, by broth microdilution method adapted from Kuete et al. [9]. A total of 8 columns were set for testing 8 different concentrations of oils ranging from 100% to 0.78% [10]. The experiment was conducted in triplicates. A total of 95μl of oil and 5μl of the standardized bacteria suspension (106 CFU/ml) were added to each well to give a final concentration of 1 x 105 CFU/ml. Wells containing 5μl of bacterial suspensions and 95μl of growth medium served as negative controls. In contrast, 5μl of the bacterial suspension mixed with 95μl of 0.12% CHX was served as a positive control [9]. A solvent control test was performed to study the effect of 0.1% ethanol on the growth of each tested bacterium. Non-inoculated wells containing 2-fold serial dilution oils served as blank controls and used for comparison with the test wells. The specimens were incubated anaerobically at 37°C for 24 hours for both species.
Following incubation, the MIC was determined by comparing the absorbance of the suspension in the wells of test extract with that of the corresponding blank. The lowest concentration of oil that showed almost similar turbidity with the blank control was recorded as the MIC value. The absorbance was measured using the ELISA reader (Varioskan Flash® Multimode Reader Thermo, USA) at wavelength 590 nm. The mean values and standard deviations were calculated for comparisons of MIC between and within groups [9].
MBC was determined by sub-culturing 10μl suspension from each well that showed almost no turbidity as well as from negative and positive controls. Then, the solution was inoculated on an agar plate and incubated at 37°C for 24 hours. The lowest concentration that exhibited an absence of growth after this sub-culturing was taken as the MBC value [9].
Transmission Electron Microscopy (TEM)
The specimens that exhibited MIC were pipetted out, harvested and prepared according to the methods by Wang et al. [11]. The prepared specimens were then observed under a transmission electron microscope (TEM) (Philips CM12 120kV, The Netherlands; Thermoscientific Talos L120C, USA).
Antiadherence effect of oils for single- and dualspecies biofilms
The antiadherence method was adapted from Kwasny, et al. [12]. The wells were first pretreated with 200μl of sterilised SWS which was extracted from a healthy volunteer with no apparent oral health disease and prepared according to Sa ́ NC et al. [13]. Then, 200μl of VOO and VCO of MIC concentration, 0.12% CHX (positive control) and sterile distilled water (negative control) were added to the individual wells in triplicates, respectively. A total of 200 μl of standardized bacterial suspension (106 CFU/ ml) of S. sobrinus (OD590 nm of 0.026) and L. casei (OD590 nm of 0.032) were later added to the test wells and incubated at 37°C for 24 hours to allow biofilm formation. The wells were then stained with crystal violet dye and incubated for 30 minutes at room temperature to quantify the amount of biofilm retained after each treatment. Next, 200 μl of 95% (v/v) ethanol was added to the wells to dissolve the biofilm. The turbidity of the biofilm suspensions in each well was determined at an optical density of 590 nm using ELISA reader (Thermo Scientific, USA). The values obtained were considered as the index of bacteria adhering to the walls of the wells in biofilm development.
Adhesion inhibition formation was calculated as below [12].
OD600(Compound)/OD600(negative control) × 100
The same experiment was duplicated using the subMIC concentration of the tested oils. As for dual species, the experiment was repeated with the standardized suspensions of the two bacteria mixed (1:1 v/v) before the experiment.
Antibiofilm effect of oils for single- and dual-species biofilms
The antibiofilm method was also adapted from Kwasny, et al. [12]. However, in contrast to the antiadherence method, a 24-hour biofilm was developed before treatment with tested oils. Following the formation of the biofilm, the wells were prepared, tested and remaining biofilm quantified in a similar manner using the same formula. The success of anti-adherence and antibiofilm agents results in declination of biofilm mass. Thus, the formula can be used for both as it quantifies the percentage of biofilm formation or survival, that measures the remaining amounts of bacterial cells and extracellular matrix under static conditions in microtiter assay plates after treatment [12]. The experiment was also repeated with subMIC concentration of the tested oils and for dual-species bacteria.
Data processing and statistical analysis
The initial data was in optical density (OD) unit, thus, to analyses the inhibition, it was calculated and expressed as mean and standard deviation. The Shapiro-Wilk test was used to test assumptions of normality. The data obtained were tabulated and analyzed using parametric statistical analysis (Statistical Package for the Social Science (IBM-SPSS®) Statistic Version 23.0) One-way between-groups analysis of variance (ANOVA) was used to compare MIC, antiadherence and antibiofilm activities between the four independent groups. The significant level was set at α=0.05. Further analysis was done using Post hoc Tukey’s HSD for multiple comparisons and Independent Samples t-Test to compare between two independent groups.
Results
Minimal inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of oils.
Table 1.1 shows MIC for VCO and VOO. VCO exhibited higher antibacterial activities against S. sobrinus and L. casei, with MIC values of 1.56% and 3.13%, respectively. Whereas, VOO showed lower inhibitory activities for S. sobrinus (6.25%) and L. casei (12.50%). Incubation of the assay plates for 24 hours was enough for all negative controls to show significant microbial growth, whereas, the wells with the positive control (0.12% CHX) (v/v) remained clear with no observable colony formation.
| Group | Bacteria | Concentration (MIC%) | OD590nm (Mean ± SD) | p-value (ANOVA) |
|---|---|---|---|---|
| VCO | S. sobrinus | 1.56 | (0.25 ± 0.03) | <0.001* |
| VOO | 6.25 | (0.30 ± 0.02) | ||
| CHX (positive control) | 0.12 | (0.01 ± 0.01) | ||
| BHI (negative control) | - | (0.61 ± 0.01) | ||
| VCO | L. casei | 3.13 | (0.26 ± 0.04) | <0.001* |
| VOO | 12.5 | (0.32 ± 0.02) | ||
| CHX (positive control) | 0.12 | (0.01 ± 0.01) | ||
| MRS (negative control) | - | (0.65 ± 0.01) |
Table 1.1: The MIC of VCO and VOO against S. sobrinus and L. casei.
The antibacterial effects of VOO and VCO against S. sobrinus were comparable with 0.12% CHX (p=0.09 and p=0.09, respectively). However, VOO exhibited significantly lower antibacterial activity towards L. casei compared to CHX (p= 0.04) (Table 1.2). The MBC for VCO and VOO were higher than the MIC where the value was 100% concentration. This indicated that both oils were only bactericidal against S. sobrinus and L. casei at the maximum concentration (100%) (Figure 1). The MBC of VCO against S. sobrinus and L. casei were 6 and 5 times higher compared to their MIC, respectively. However, VOO exhibited low bactericidal effects against S. sobrinus and L. casei. They were only 4 and 3 times higher than their MIC, respectively.
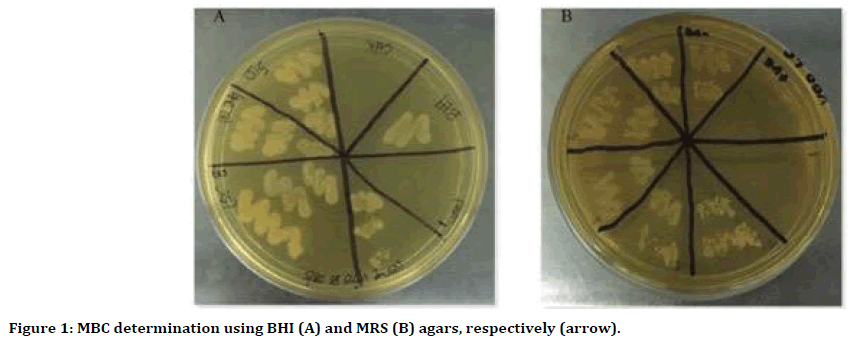
Figure 1. MBC determination using BHI (A) and MRS (B) agars, respectively (arrow).
| Dependent Variable (Bacteria) | (Sample) Group | Mean±SD (OD 550nm) | Compared with | Mean ± SD (OD 550nm) | p-value (Tukey’s post hoc test) |
|---|---|---|---|---|---|
| S. sobrinus | CHX (0.12%) | 0.01 ± 0.01 | VCO | 0.25 ± 0.03 | 0.09 |
| VOO (6.25%) | 0.30 ± 0.02 | 0.11 | |||
| VCO (-1.56%) | 0.25 ± 0.03 | VOO | 0.30 ± 0.02 | 0.4 | |
| L. casei | CHX (0.12%) | 0.01 ± 0.01 | VCO | 0.26 ± 0.04 | 0.09 |
| VOO (12.50%) | 0.32 ± 0.02 | 0.04* | |||
| VCO (-3.13%) | 0.26 ± 0.04 | VOO | 0.32 ± 0.02 | 0.34 |
Table 1.2: The MIC of VCO and VOO against S. sobrinus and L. casei.
Morphological changes
Without any treatment, the surface of the cell walls of S. sobrinus and L. casei colonies appeared smooth with intact cell membranes and complete cell content (Figure 2A and 3A). Significant cytological differences were observed in these bacteria after exposure to VCO and VOO, respectively. Extracellular and intracellular destructions were evident for both bacteria under TEM (Figure 2B-D and 3B-D). Cytoplasm separation from the cell wall was apparent after treatment with 0.12% CHX (Figure 2B and 3B). The ultrastructural changes of damaged S. sobrinus cells showed destroyed, thickened cell walls and disturbed cell division (Figure 2CD). Besides, TEM photomicrographs of L. casei after treatment with both oils showed the cellular contents leaked out through the porous wall and subsequently resulted in cell lysis (Figure 2C-D and 3C-D). The formation of cystic spaces was also seen within the damaged cells (Figure 3C-D).
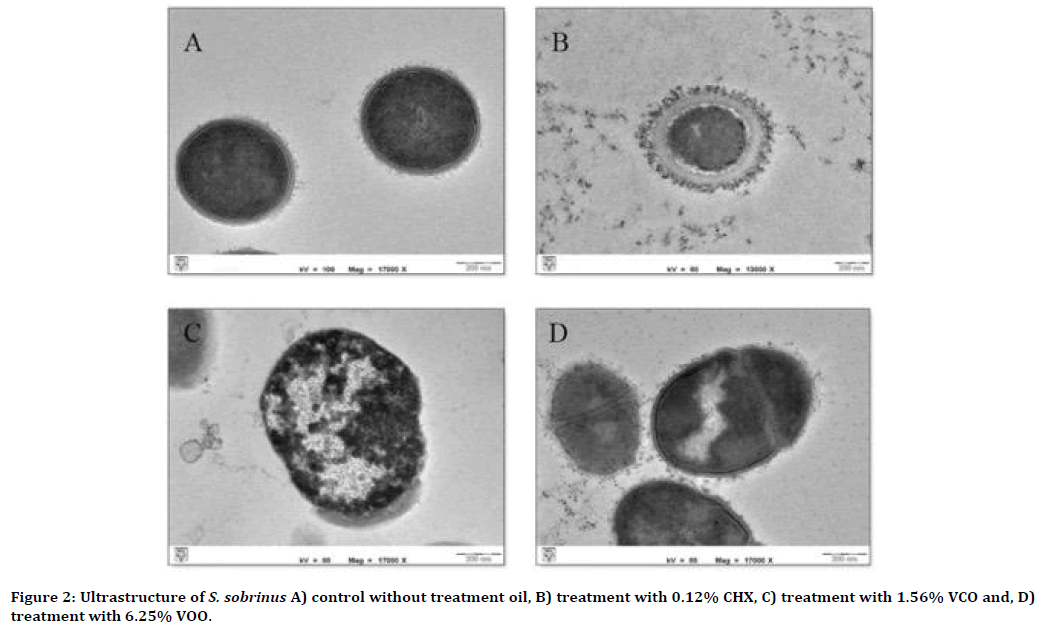
Figure 2. Ultrastructure of S. sobrinus A) control without treatment oil, B) treatment with 0.12% CHX, C) treatment with 1.56% VCO and, D) treatment with 6.25% VOO.
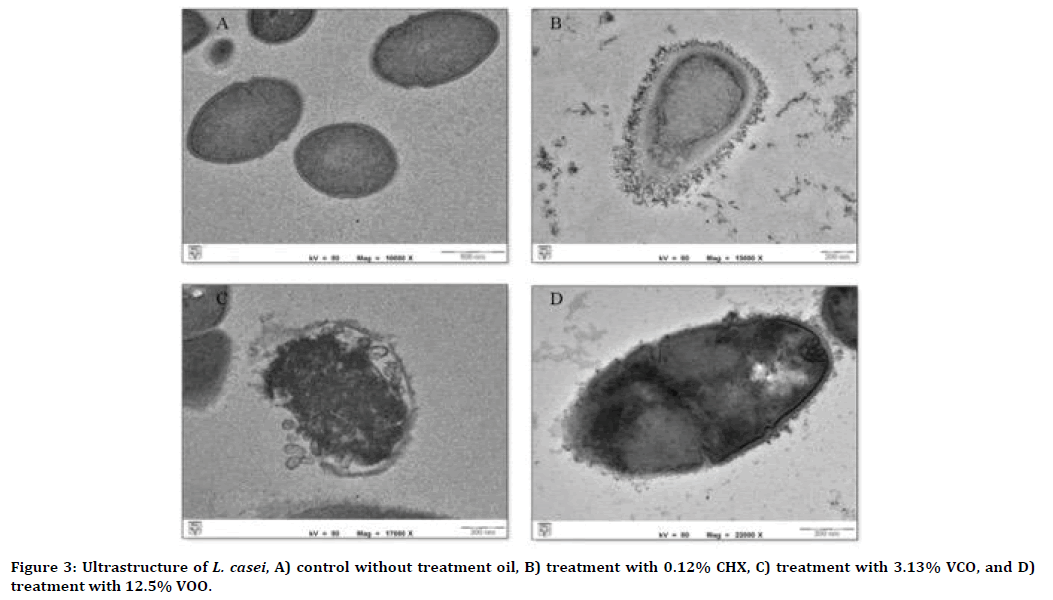
Figure 3. Ultrastructure of L. casei, A) control without treatment oil, B) treatment with 0.12% CHX, C) treatment with 3.13% VCO, and D) treatment with 12.5% VOO.
Antiadherence effect of oils for single- and dualspecies biofilms
Generally, the ant adhering effect of both oils was higher than the positive control, 0.12% CHX (Table 2). For single and dual-species biofilms, VOO demonstrated significantly higher antiadherence activity than 0.12% CHX (all p<0.001, respectively) (Table 3). Conversely, there was no statistically significant difference in antiadherence effect between VCO and 0.12% CHX towards single-species biofilm of S. sobrinus and L. casei (p=0.07 and p=0.06, respectively) (Table 3, Figure 4). Table 4 shows the antiadherence effect of VCO and VOO at the subMIC level. The result revealed that all VCO and VOO at subMIC level was able to inhibit the adherence of S. sobrinus, L. casei and dualspecies biofilms (Table 4). However, both oils showed significantly lower antiadherence activity towards single- and dual-species biofilms as compared to 0.12% CHX (all p< 0.001, respectively) (Table 5, Figure 5).
| MIC of sample | Percentage of antiadherence (Mean ± SD) (%) | |||
|---|---|---|---|---|
| S. sobrinus | L. casei | Dual-species biofilm | ||
| VCO | 74.67 ± 0.58 | 70.33 ± 1.53 | 68.00 ± 1.01 | |
| VOO | 80.67 ± 2.08 | 75.33 ± 1.53 | 73.00 ± 2.01 | |
| 0.12% CHX | 72.67 ± 2.52 | 67.67 ± 1.53 | 61.00 ± 1.01 | |
| Analysis of variance | F | 831.12 | 129.39 | 728.54 |
| (ANOVA) | p- value | <0.001* | <0.001* | <0.001* |
Table 2: Antiadherence activity of VCO, VOO and 0.12% CHX.
| Dependent Variable | Group | Compared with | (Mean difference ± SE) (%) | p-value (Tukey’s post hoc test) |
|---|---|---|---|---|
| S. sobrinus | CHX | VCO | 2.00 ± 1.17 | 0.07 |
| VOO | 8.00 ± 1.17 | <0.001* | ||
| VCO | VOO | 6.00 ± 1.17 | 0.01* | |
| L. casei | CHX | VCO | 2.66 ± 1.17 | 0.06 |
| VOO | 7.66 ± 1.17 | <0.001* | ||
| VCO | VOO | 5.00 ± 1.17 | 0.03* | |
| Dual-species biofilm | CHX | VCO | 7.00 ± 1.17 | <0.001* |
| VOO | 12.00 ± 1.17 | <0.001* | ||
| VCO | VOO | 5.00 ± 1.17 | 0.03* |
Table 3: Between-group comparisons of antiadherence activity of oils against single- and dual-species biofilms.
| SubMIC of sample | Percentage of bacteria antiadherence (Mean ± SD) (%) | |||
|---|---|---|---|---|
| S. sobrinus | L. casei | Dual-species biofilm | ||
| VCO | 16.67 ± 1.53 | 12.67 ± 0.40 | 8.33 ± 0.80 | |
| VOO | 20.33 ± 2.01 | 16.67 ± 1.53 | 11.33 ± 2.50 | |
| 0.12% CHX | 72.67 ± 2.52 | 67.67 ± 1.53 | 61.00 ± 1.01 | |
| Analysis of variance | F | 612.53 | 604.05 | 520.54 |
| (ANOVA) | p- value | <0.001* | <0.001* | <0.001* |
Table 4: Antiadherence activity of subMIC VCO, VOO and 0.12% CHX.
| Dependent Variable | Group | Compared with | (Mean difference ± SE) (%) | p-value (Tukey’s post hoc test) |
|---|---|---|---|---|
| S. sobrinus | CHX | VCO | 56.00 ± 1.22 | <0.001* |
| VOO | 52.34 ± 1.22 | <0.001* | ||
| VCO | VOO | 3.66 ± 1.22 | 0.23 | |
| L. casei | CHX | VCO | 55.00 ± 1.22 | <0.001* |
| VOO | 51.00 ± 1.22 | <0.001* | ||
| VCO | VOO | 4.00 ± 1.22 | 0.08 | |
| Dual-species biofilm | CHX | VCO | 52.67 ± 1.22 | <0.001* |
| VOO | 49.67 ± 1.22 | <0.001* | ||
| VCO | VOO | 3.00 ± 1.22 | 0.2 |
Table 5: Between-group comparisons of antiadherence activity of oils against single- and dual-species biofilms.
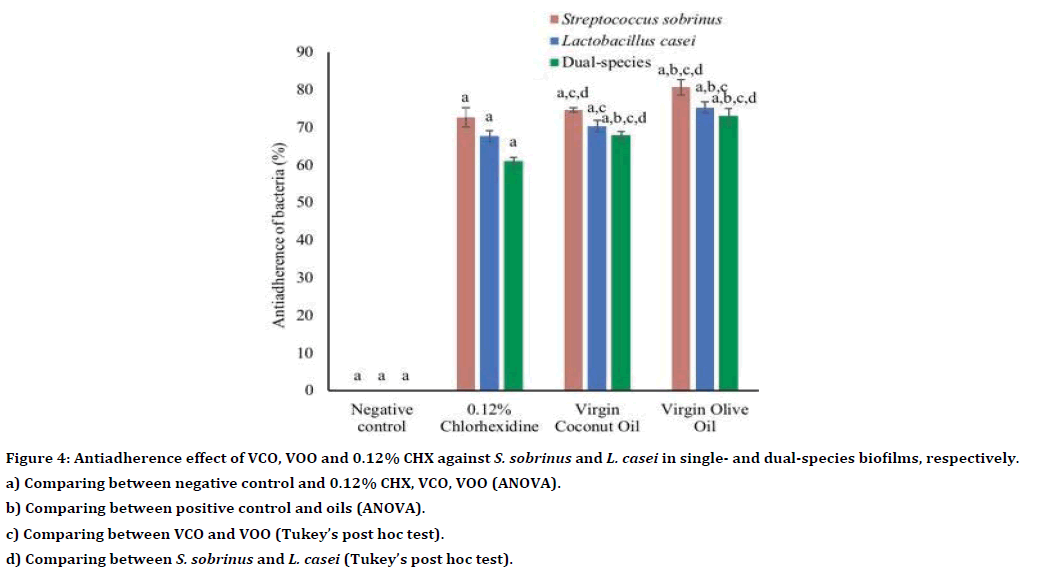
Figure 4. Antiadherence effect of VCO, VOO and 0.12% CHX against S. sobrinus and L. casei in single- and dual-species biofilms, respectively.
a) Comparing between negative control and 0.12% CHX, VCO, VOO (ANOVA).
b) Comparing between positive control and oils (ANOVA).
c) Comparing between VCO and VOO (Tukey’s post hoc test).
d) Comparing between S. sobrinus and L. casei (Tukey’s post hoc test).
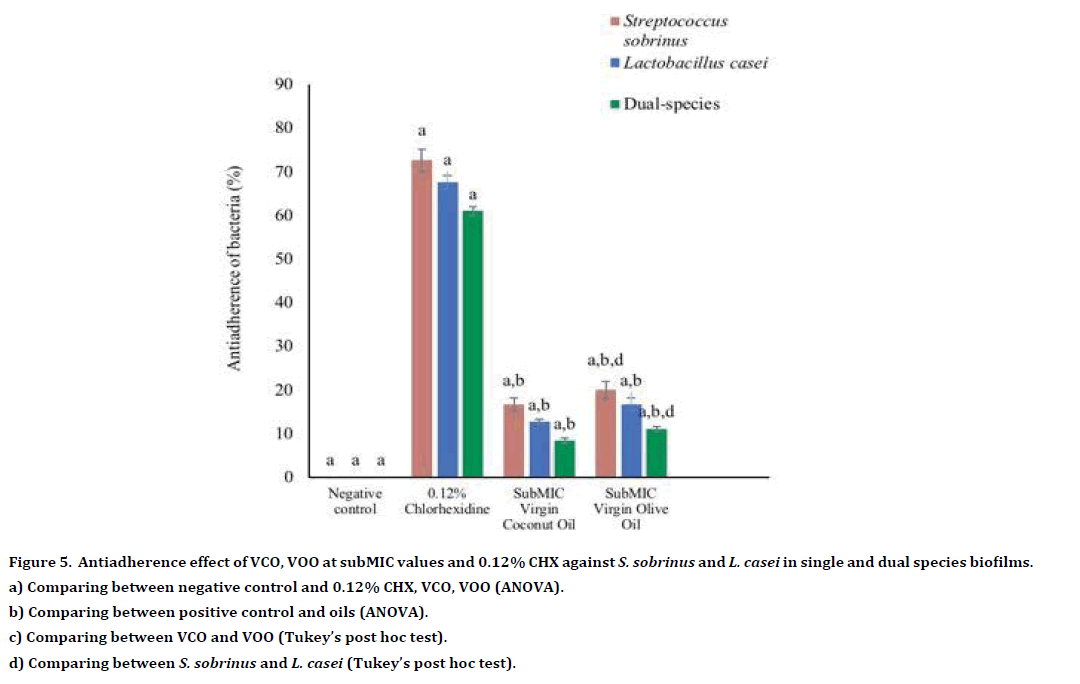
Figure 5. Antiadherence effect of VCO, VOO at subMIC values and 0.12% CHX against S. sobrinus and L. casei in single and dual species biofilms.
a) Comparing between negative control and 0.12% CHX, VCO, VOO (ANOVA).
b) Comparing between positive control and oils (ANOVA).
c) Comparing between VCO and VOO (Tukey’s post hoc test).
d) Comparing between S. sobrinus and L. casei (Tukey’s post hoc test).
Antibiofilm effect of oils for single and dual-species biofilms
Both VCO and VOO demonstrated antibiofilm activity towards single- and dual-species biofilms of S. sobrinus and L. casei (Table 6). Generally, VCO showed higher antibiofilm activity compared to VOO and CHX (Table 6). Compared to the positive control (0.12% CHX), VCO exhibited significantly higher antibiofilm activity towards single species of S. sobrinus (p<0.001) and L. casei (p<0.001) and dual species (p<0.001). Whereas, the antibiofilm effect of VOO was like 0.12% CHX towards S. sobrinus and L. casei in single-species biofilm, p=0.1.00, p=0.79 and p=0.06, respectively (Table 7, Figure 6). At the subMIC level, both oils showed antibiofilm activity towards S. sobrinus, L. casei and dualspecies biofilms (Table 8). However, compared to 0.12% CHX, VCO and VOO at subMIC showed significantly lower antibiofilm activity against all tested bacteria (all p<0.001, respectively) (Table 9). Also, there was no significant difference between the antibiofilm effect of both oils towards single-species biofilm and dual-species biofilms (p>0.05) (Table 9, Figure 7).
| MIC of sample | Percentage of antibiofilm (Mean ± SD) (%) | |||
|---|---|---|---|---|
| S. sobrinus | L. casei | Dual-species biofilm | ||
| VCO | 60.67 ± 1.01 | 56.33 ± 1.10 | 50.33 ± 2.10 | |
| VOO | 55.00 ± 2.08 | 52.67 ± 1.53 | 37.67 ± 1.56 | |
| 0.12% CHX | 54.67 ± 1.53 | 50.67 ± 1.60 | 33.67 ± 1.60 | |
| Analysis of variance | F | 429.63 | 723.18 | 736.69 |
| (ANOVA) | p-value | <0.001* | <0.001* | <0.001* |
Table 6: Antibiofilm activity of VCO, VOO and 0.12% CHX.
| Dependent Variable | Group | Compared with | (Mean difference ± SE) (%) | p-value (Tukey’s post hoc test) |
|---|---|---|---|---|
| S. sobrinus | CHX | VCO | 6.00 ± 1.15 | <0.001* |
| VOO | 0.33 ± 1.15 | 1 | ||
| VCO | VOO | 5.67 ± 1.15 | <0.001* | |
| L. casei | CHX | VCO | 5.66 ± 1.15 | <0.001* |
| VOO | 2.00 ± 1.15 | 0.79 | ||
| VCO | VOO | 3.66 ± 1.15 | 0.11 | |
| Dual-species biofilm | CHX | VCO | 16.66 ± 1.15 | <0.001* |
| VOO | 4.00 ± 1.15 | 0.06 | ||
| VCO | VOO | 12.66 ± 1.15 | <0.001* |
Table 7: Between-group comparisons of antibiofilm activity of oils against single- and dual-species biofilms.
| Percentage of biofilm formation inhibition (Mean ± SD) (%) | ||||
|---|---|---|---|---|
| S. sobrinus | L. casei | Dual-species biofilm | ||
| VCO | 10.67 ± 1.01 | 8.33 ± 1.01 | 5.33 ± 0.50 | |
| VOO | 4.33 ± 0.20 | 2.67 ± 0.40 | 1.33 ± 0.10 | |
| 0.12% CHX | 54.67 ± 1.53 | 50.67 ± 1.60 | 33.67 ± 1.60 | |
| Analysis of variance (ANOVA) | F p- value | 459.43 | 911.18 | 636.44 |
| <0.001* | <0.001* | <0.001* | ||
Table 8: Antibiofilm activity of subMIC VCO, VOO and CHX.
| Dependent Variable | Group | Compared with | (Mean difference ± SE) (%) | p-value (Tukey’s post hoc test) |
|---|---|---|---|---|
| S. sobrinus | CHX | VCO | 44.00 ± 1.01 | <0.001* |
| VOO | 50.34 ± 1.01 | <0.001* | ||
| VCO | VOO | 6.34 ± 1.01 | 0.07 | |
| L. casei | CHX | VCO | 42.34 ± 1.01 | <0.001* |
| VOO | 48.00 ± 1.01 | <0.001* | ||
| VCO | VOO | 5.66 ± 1.01 | 0.08 | |
| Dual-species biofilm | CHX | VCO | 28.34 ± 1.01 | <0.001* |
| VOO | 32.34 ± 1.01 | <0.001* | ||
| VCO | VOO | 4.00 ± 1.01 | 0.08 |
Table 9: Between-group comparisons of antibiofilm activity of oils against single- and dual-species biofilms.
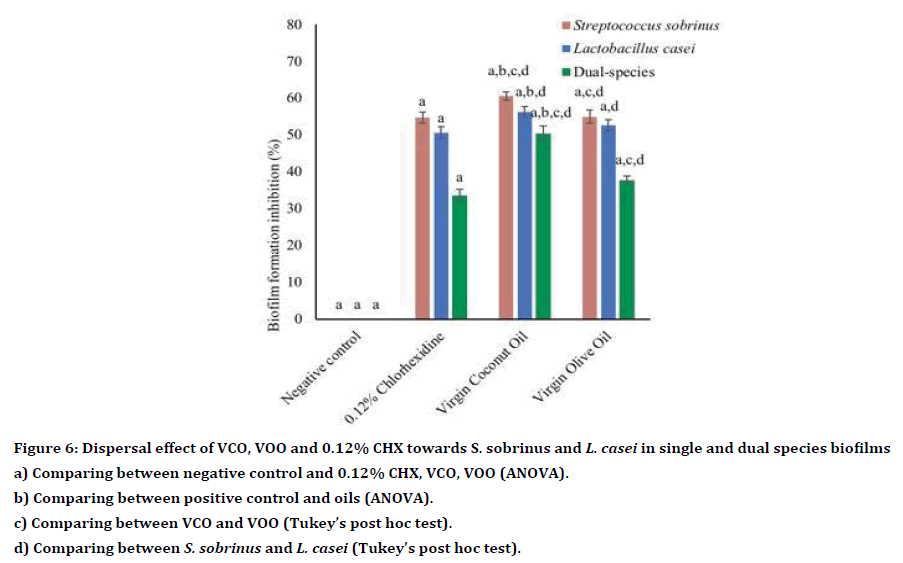
Figure 6. Dispersal effect of VCO, VOO and 0.12% CHX towards S. sobrinus and L. casei in single and dual species biofilms
a) Comparing between negative control and 0.12% CHX, VCO, VOO (ANOVA).
b) Comparing between positive control and oils (ANOVA).
c) Comparing between VCO and VOO (Tukey’s post hoc test).
d) Comparing between S. sobrinus and L. casei (Tukey’s post hoc test).
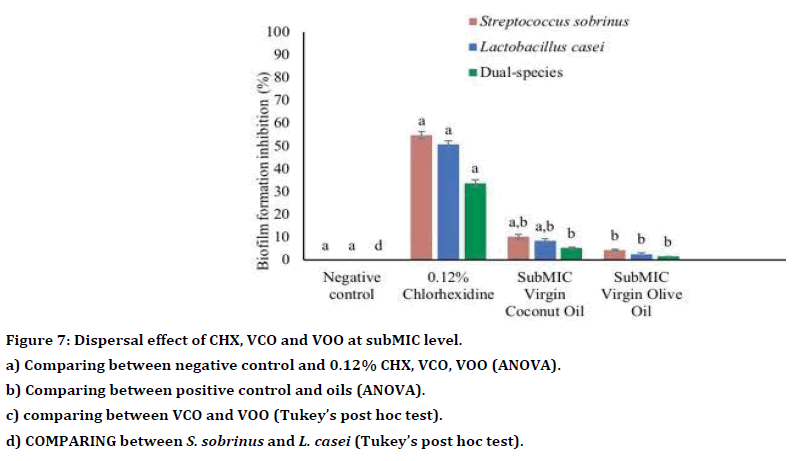
Figure 7. Dispersal effect of CHX, VCO and VOO at subMIC level.
a) Comparing between negative control and 0.12% CHX, VCO, VOO (ANOVA).
b) Comparing between positive control and oils (ANOVA).
c) comparing between VCO and VOO (Tukey’s post hoc test).
d) COMPARING between S. sobrinus and L. casei (Tukey’s post hoc test).
Discussion
In this study, both VCO and VOO were shown to have antibacterial activity against S. sobrinus and L. casei VCO exhibited higher antibacterial activity against both bacteria, whereas, VOO showed lower inhibitory activity for S. sobrinus and L. casei. The findings were like those obtained in a study conducted on other organisms such as Helicobacter pylori, Staphylococcus aureus, S. mutans, and Candida albicans which demonstrated that VCO had antimicrobial activity against the tested microorganisms [10,14]. A randomized clinical study focused on a low-income population also found a significant improvement in oral hygiene measures in all groups treated with sesame, olive and coconut oils and CHX gel [15]. Recent studies have also shown that oil pulling with VCO can reduce oral microorganisms particularly S. mutants [16,17]. These findings agree with our study, in which, VOO and VCO were found to inhibit the growth of S. sobrinus and L. casei.
VCO is a rich source of beneficial medium-chain fatty acids (MCFAs), mainly lauric acid, capric acid, caprylic acid and caproic acid. Lauric acid is proven to exhibit the most potent antibacterial activity against mainly Gram-positive bacteria, including Staphylococcus aureus; a major pathogen for skin infection [18]. A high phenolic composition (tyrosol, and hydroxytyrosol) contributes to the biochemical characteristics of VOO, particularly in its antibacterial activity [19]. Hence, we believe that the antibacterial effect against tested bacteria as reported in the present study might be linked to the presence of fatty acid or phenolic constituents of the oils.
The in vitro data obtained from this study showed that VCO has higher antibacterial activity at a lower concentration compared to VOO. According to the study by Anzaku et al. even at the lowest diluted concentration, lauric acid exerts a relatively similar antibacterial effect against Staphylococcus aureus, Streptococci, and Lactobacilli. Furthermore, VCO at its lowest dilution concentration was more effective against Gram-positive bacteria but relatively less effective against Gram-negative bacteria [20].
Both oils showed similar efficacy against S. sobrinus but VOO showed slightly lower antibacterial activity against L. casei. It has been reported that lactic acid bacteria such as L. casei have developed an intrinsic resistance mechanism towards aminoglycoside type antibiotics. The genes aac (3) and lsa found in Lactobacillus sp, have been postulated to be responsible for resistance towards aminoglycosides and clindamycin, respectively [21]. Therefore, there is a possibility that L. casei might have developed a similar mechanism of resistance towards VOO, consequently, the lower antibacterial activity as compared to VCO.
The TEM images of S. sobrinus and L. casei cells showed significant changes to their cytoplasm as well as their external appearance after treatment with VCO. These changes might be associated with alterations in membrane permeability as demonstrated in a study of another natural oil i.e Copaiba oil [22]. The hydrophobic properties of oil may alter cell membrane permeability to water and ions that could then lead to organelle disintegration, cystic and spore formation, and subsequently, to cell death [22].
To the best of our knowledge, the antiadherence activity of VCO and VOO has not been previously reported. Traditionally, antibacterial agents are referred to as materials that can cause bacterial death. However, in recent years, it is also considered acceptable to develop antibacterial materials that can minimize bacterial adhesion rather than killing the bacteria directly, thus, the shift in the manufacturing trend [23]. In the present study, VOO demonstrated the highest antiadherence activity compared to 0.12% CHX and VCO. Likewise, in-vivo experiments have also shown that olive oil (both alone and in the paste form) caused plaque inhibition by up to 22% [24]. They claimed that any agent that can reduce plaque by at least 20% can be considered as an effective antiadherence agent. VCO is also an effective emulsifier and has a high saponification value. It was found to reduce plaque adhesion by its interaction with alkalis, such as bicarbonates in saliva. VCO can initiate the formation of a soap-like substance which greatly enhances the surface area of the oil that would eventually result in reduced plaque adhesion [15].
The antiadherence activity may be associated with super hydrophobicity of the tested materials when the water contact angle is more than 150º [23]. Based on the findings, removal of bacteria is easier as superhydrophobic material can reduce the adhesion force between bacteria and a solid surface, thus preventing the formation of thick biofilm [23]. Hence, the use of these oils as a mouthwash would be beneficial for people with poor manual dexterity or have a physical disability by facilitating plaque removal on top of reducing bacterial adhesion on the tooth surface.
Even though subMIC concentration does not usually kill bacteria, the presence of a certain number of antibacterial components in the oil may inhibit bacterial growth. Thus, in this study, subMIC concentration was utilized as it might show antiadherence activity at subMIC levels and subsequently affect the biofilm formation. An earlier report showed antibiotics at subMIC level can harm bacterial growth and able to modify bacteria biochemistry at concentrations below MIC [25]. Therefore, in this study, MIC and subMIC concentrations were utilized to observe the effect of oils on bacterial adherence and biofilm formation.
Bacteria in biofilms are commonly found in dualand multispecies form rather than the planktonic state. For that reason, we utilized dual species in our experiment to imitate the biofilm effect and replicate the oral environment. For an antimicrobial to exert its effect, it needs to diffuse into the deepest layer of the biofilm which can be prevented by a primary barrier formed by the extracellular DNA within the matrix, compaction of exopolysaccharide matrix and complex biofilm structure. This phenomenon has contributed to the poor penetration of CHX molecules into the biofilm of both single- and dual-species [26]. Compared to an essential oil containing mouthwash, the effectiveness of CHX against the biofilm of Streptococcus mutans, Fusobacterium nucleatum and mixed bacteria is dose-dependent [27]. This could explain the reduced antibiofilm effects of CHX, as well as both oils against dualspecies biofilms at MIC and subMIC values in our study.
In this study, VCO showed the highest antibiofilm activity against single- and dual-species biofilms. It may be suggested that compounds within VCO not only displayed antibacterial but also antibiofilm action as well. As proposed by a recent study, lauric acid, an unsaturated fatty acid contributes to high antibiofilm activity by inhibiting microbial survival and biofilm growth of S. Mutans [20].
To date, the antibiofilm activity of VOO has not been reported. However, the antibiofilm effect of most natural based products is mainly due to interruption of matrix formation, inhibition of cellular adhesion and communication, and decreasing virulence factors production, thereby blocking biofilm development [28]. Thus, this could explain antibiofilm activity observed in both oils against single- and dual-species biofilms.
In antiadherence and antibiofilm activities, bacteria inhibition was higher in single-species compared to dual-species for both MIC and subMIC concentrations. This suggests an increase in the resistance provided by dual-species biofilms. A pronounced reduction in inhibition by oils at subMIC concentration against dualspecies biofilms as compared to 0.12% CHX. The enhanced resistance to VCO and VOO may result from the complexity of dual-species biofilm matrix which impairs the diffusion of inhibitory compounds in the oils [29].
Conclusion
This study was a preliminary evaluation of antibacterial, antiadherence and antibiofilm effects of VCO and VOO. In conclusion, the antibacterial effect of VCO and VOO is as effective as 0.12% CHX. Both oils show potential as alternative mouthwashes in dental caries prevention. The effectiveness of VOO is at the initiation stage of plaque formation. However, once plaque has formed, the use of VCO based mouthwash is advisable due to its superior antibiofilm activity.
References
- Duangthip D, Gao SS, Lo EC, et al. Early childhood caries among 5-6-year-old children in Southeast Asia. Int Dent J 2016; 67:98-106.
- Petersen PE. Challenges to improvement of oral health in the 21st century the approach of the WHO global oral health programme. Int Dent J 2004; 54:329–43.
- Thomas A, Thakur SR, Shetty SB. Anti-microbial efficacy of green tea and chlorhexidine mouth rinses against Streptococcus mutans, Lactobacilli spp and Candida albicans in children with severe early childhood caries: A randomized clinical study. J Indian Soc Pedod Prev Dent 2016; 34:65-70.
- Ragul P, Dhanraj M, Ashish RJ. Efficacy of eucalyptus oil over chlorhexidine mouthwash in dental practice. Drug Inv Today 2018; 10:5.
- Akinkunmi EO, Lamikanra A. Susceptibility of community associated methicillin resistant Staphylococcus aureus isolated from faeces to antiseptics. J. Infect Dev Ctries 2012; 6:317–324.
- Aprile MC, Caputo V, Nayga RM. Consumers’ preferences and attitudes toward local food products. J Food Prod Mark 2016; 22:19–42.
- Yap PSX, Yiap BC, Ping HC, et al. Essential oils, a new horizon in combating bacterial antibiotic resistance. Open Microbiol J 2014; 8:6–14.
- Chaudhari LKD, Jawale BA, Sharma S, et al. Antimicrobial activity of commercially available essential oils against Streptococcus mutans. J Contemp Dent Pract 2012; 13:71–74.
- Kuete V, Fozing DC, Kapche WFGD, et al. Antimicrobial activity of the methanolic extract and compounds from Morus mesozygia stem bark. J Ethnopharmacol 2009; 124:551–555.
- Ogbolu DO, Oni AA, Daini OA, et al. In vitro antimicrobial properties of coconut oil on Candida species in Ibadan, Nigeria. J Med Food 2007; 10:384–387.
- Wang W, He J, Pan D, et al. Metabolomics analysis of Lactobacillus plantarum ATCC 14917 adhesion activity under initial acid and alkali stress. PLoS ONE 2018; 13:1–16.
- Kwasny SM, Opperman TJ. Static biofilm cultures of gram-positive pathogens grown in a microtiter format used for anti-biofilm drug discovery. Current Protocols Pharmacol 2010; Chapter 13.
- Sa ́ NC, Thays T, Cavalcante A, et al. Antimicrobial and antibiofilm action of casbane diterpene from croton nepetaefolius against oral bacteria. Arch Oral Bio 2011; 7:550–555.
- Meng J, Chen T, Chang Y, et al. Study of the mechanism of anti-ulcer effects of virgin coconut oil on gastric ulcer-induced rat model. Arch Med Sci 2019; 15:1329-1335.
- Singla N, Acharya S, Martena S, et al. Effect of oil gum massage therapy on common pathogenic oral microorganisms-A randomized controlled trial. J Indian Soc Periodontol 2014; 18:441–446.
- Peedikayil FC, Remy V, John S, et al. Comparison of antibacterial efficacy of coconut oil and chlorhexidine on Streptococcus mutans: An in vivo study. J Int Soc Prevent Communit Dent 2016; 6(5):447–452.
- Shanbhag VKL. Oil pulling for maintaining oral hygiene–A review. J Tradit Complement Med 2017; 7:106–109.
- Yoon BK, Jackman JA, Valle-González ER, et al. Antibacterial free fatty acids and monoglycerides: Biological activities, experimental testing, and therapeutic applications. Int. J Mol Sci 2018; 19:1–40.
- Daǧdelen A. Identifying antioxidant and antimicrobial activities of the phenolic extracts and mineral contents of virgin olive oils (Olea europaea L. cv. Edincik Su) from different regions in Turkey. J Chem 2016: 1–11.
- Anzaku AA, Assikong EB, Akeh M, et al. Antimicrobial Activity of coconut oil and its derivative (lauric acid) on some selected clinical isolates. Int J Med Sci Clin Invent 2017; 4:3173–3177.
- Campedelli I, Mathur H, Salvetti E, et al. Genus-wide assessment of antibiotic resistance in Lactobacillus spp. Appl Environ Microbiol 2019; 85:1–21.
- Nakamuraa MT, Endo EH, de Sousa JPB, et al. Copaiba oil and its constituent copalic acid as chemotherapeutic agents against dermatophytes. J Brazillian Chem Soc 2017; 28:1377–1383.
- Zhang X, Wang L, Leva E. Superhydrophobic surfaces for the reduction of bacterial adhesion. RSC Adv 2013; 3:12003–12020.
- Pretty IA, Gallagher MJ, Martin MV, et al. A study to assess the effects of a new detergent-free, olive oil formulation dentifrice in vitro and in vivo. J Dent 2003; 31:327–332.
- Tezel BU, Akçelik N, Yüksel FN, et al. Effects of sub-MIC antibiotic concentrations on biofilm production of salmonella infantis. Biotechnol Equip 2016; 30:1184–1191.
- Saleem HGM, Seers CA, Sabri AN, et al. Dental plaque bacteria with reduced susceptibility to chlorhexidine are multidrug resistant. BMC Microbiol 2016; 16:1–9.
- Solmaz G, Korachi M. Inhibition and disruption properties of chlorhexidine gluconate on single and multispecies oral biofilms. Jundishapur J Microbiol 2013; 6:61–66.
- Lu L, Hu W, Tian Z, et al. Developing natural products as potential anti-biofilm agents. Chinese Med 2019; 14:11.
- Burmølle M, Webb JS, Rao D, et al. Enhanced biofilm formation and increased resistance to antimicrobial agents and bacterial invasion are caused by synergistic interactions in multispecies biofilms. Appl. Environ. Microbiol 2006; 72:3916–3923.
Author Info
Nur Hafizah Hazmi1, Zaleha Shafiei2, Ahmad Shuhud Irfani Zakaria1, S Nagarajan MP Sockalingam1 and Alida Mahyuddin1*
1Department of Family Oral Health, Faculty of Dentistry, University Kebangsaan Malaysia, Malaysia2Department of Craniofacial Diagnostics and Biosciences, Faculty of Dentistry, University Kebangsaan Malaysia, Malaysia
Citation: Nur Hafizah Hazmi, Zaleha Shafiei, Ahmad Shuhud Irfani Zakaria, S Nagarajan MP Sockalingam, Alida Mahyuddin, Antibacterial and Antibiofilm Effects of Virgin Coconut and Virgin Olive Oils on Streptococcus sobrinus and Lactobacillus casei, J Res Med Dent Sci, 2020, 8 (3):141-152.
Received: 09-May-2020 Accepted: 18-May-2020
