Research - (2021) Volume 9, Issue 4
Antibacterial Activity of Wound Healing Spacer Materials Coated with Green Synthesized AgNP-AMP Conjugates against âESKAPEâ Members of Clinical Isolates from Different Types of Wounds
Kuppannan Gobianand1, Muhammad Musthafa1* and Manohar M2
*Correspondence: Muhammad Musthafa, Department of Microbiology, Vivekanandha College of Arts and Sciences for Women, India, Email:
Abstract
Different types of wounds and the complications arising from them including multi-drug resistant bacterial infections cause prolonged morbidities and high rates of mortalities resulting in worsening economic stability and hospital systems in developing countries. The management of wounds is a complex process as they provide typical environment for the colonization of contaminated microorganisms including normal floral members with abundance of nutrients, optimal physical conditions, and lesser resistance by the weaker host immune system. Several different types of Gram-positive and Gram-negative bacterial species have been isolated and the infections by the so-called notorious “ESKAPE” pathogens viz. Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter species/ Escherichia coli are more serious because of their extensively drug resistance nature. Silver and its various compounds were used in the management of wounds since time unknown and the silver nanoparticles (AgNPs), in recent decades, have shown potential, promising activities against most of the drug-resistant bacterial pathogens. When conjugated with protein-based compounds like antimicrobial peptides (AMPs – which are known for their potential antimicrobial properties), AgNPs were reported to have shown enhanced activities against pathogens. So, the present study was designed to isolate and identify predominant bacterial pathogens from different types of acute and chronic wounds (as accident/trauma, diabetic wounds/ulcers and burn wounds) from the in-patients at a tertiary care hospital and to analyze their responses in in vitro to AgNPs, AMPs, AgNP-AMP conjugates and to the wound healing spacer materials coated with AgNP-AMP conjugates. The results were highly encouraging in observing that the AgNPs, AMPs and their conjugates have shown potential activities against the predominant members of the isolates. When coated with the AgNP-AMP conjugates, the wound healing spacer materials could prevent the bacterial growth with a wider inhibition zone than in any of the other cases. So, we realize and recommend that these materials should be investigated more for the benefit of the communities in developing countries.
Keywords
Wound infections, ESKAPE pathogens, Silver nanoparticles, Antimicrobial peptides, AgNP-AMP conjugates, Wound healing spacer materials
Introduction
Wounds, which are the disturbances or destructions in the structural integrity of the dermal tissues are broadly classified as acute (like trauma, burns, surgical etc.) or chronic (like pressure sores or bed sores, diabetic wounds of foot (DFUs) etc.), pose a considerable threat to the healthcare and economic system all over the world [1-3] and the infections and complications arising from such wounds are considered as one of the major causes of prolonged morbidities and high rate of mortalities in developing countries [4-6]. The management of different types of wounds is a complicated process as the wound healing has several interrelated cellular and extra-cellular phases as inflammatory, proliferative and remodeling [2,3].
As the hypodermis being exposed and the host immune system is compromised [7], the wound formation provides an optimal, favorable condition with moisture, optimal temperature, and rich nutrition [4,8] for the contaminated microorganisms to grow and colonize and to proliferate, thereby increasing the chronicity of the wound and making the healing process complicated and delayed [9,10]. Also, other factors like the site of wound, its depth, and dimensions along with the type of contaminating microbes also play an important role in deciding the fate of wound healing [6,11].
The microorganisms can reach the wound site from the environment or from the adjacent skin or from the internal environment like mucosa [7]. Thus, once the wound infection– pathogenic microbial proliferation of cutaneous and subcutaneous soft tissues and the microbes including pathogens invade into the viable, inner tissues [6,12]. When the bacterial members are of antimicrobial resistant (AMR) or multidrug resistant (MDR) strains, they bring about serious complications resulting in prolonged morbidity and high costs of healthcare [6,13]. Several bacteria including MDR strains have been isolated from such wounds and the common ones include Gram-positive bacterial species like Staphylococcus epidermidis, Staphylococcus aureus, Streptococcus pyogenes and other Strptococci and Enterococci, and Gram-negative species like Klebsiella pneumoniae, Pseudomonas spp., Escherichia coli and Proteus spp. [4,6,14]. Among these bacteria, according to the Infectious Diseases Society of America–IDSA, there are five prime members which require special attention as they pause bigger threat [15] by managing to “escape” the actions of antimicrobial agents thereby forming a group of MDR (multidrugresistant) and XDR (extensively drug-resistant) pathogens, causing two-third of all healthcareassociated infections (HAIs). They are dubbed as “ESKAPE” pathogens and are: Enterococcus faecium (E), Staphylococcus aureus (S), Klebsiella pneumoniae (K), Acinetobacter baumannii (A), Pseudomonas aeruginosa (P) and Enterobacter species/ Escherichia coli (E) [16,17].
Recorded as early as 1850 BC, in Egypt, the metal silver has been used in wound management and its advantages have been described by Hippocrates in his textbooks [2]. Even though the discovery and wide application of antibiotics made the silver usage less popular, now, in recent decades, the scientific world is analyzing metals and compounds of natural backgrounds including silver, in the wake of widespread resistance of various pathogens against antibiotics [2,18]. The potentials of silver nanoparticles (AgNPs) as a promising antimicrobial agent have been proven in several studies [16,18] and are in application level [19]. Thus, the nanoscience provides metal nanoparticles with unique mode of action for improved and fastened wound healing both in acute and chronic complications [2,19,20]. When these nanoparticles are embedded and coated with biomaterials like wound healing spacer materials, it has been noted that the overall process of wound healing is further improved [20,21].
Antimicrobial peptides (AMPs), otherwise known as "natural antibiotics" [22] are relatively short, evolutionarily conserved amino acid hydrophobic residues with an overall positive charge [23,24]. In marine invertebrates, these AMPs are the prime actors of the only defense mechanism–the innate immune system and have shown a broad spectrum of potentials against all the microorganisms including pathogens from bacteria, viruses, and fungi [25-27].
The studies on the interaction between different proteins like AMPs and silver nanoparticles have proven that, such conjugations enhance the biocompatibility of the nanomaterial thereby increasing its antimicrobial activities. Complex compounds like peptides, proteins and even free amino acids have been found to have control over the structure of the silver nanoparticles at the time of its synthesis and can improve the stability in different situation [28,29]. Also, it is interesting to note that the bio-functionalized silver nanoparticles can detoxify and remove the endotoxin, which in turn, promising their potential in various applications [30,31]. Thus, the present study was designed to isolate and identify prominent bacterial pathogens from different types of wounds from the in-patients of a tertiary hospital and to analyze the activity of wound healing spacer materials coated with green synthesized silver nanoparticleantimicrobial peptide (AgNP-AMP) conjugates against these isolates.
Materials and Methods
Extraction, green synthesis, and characterization of AgNPs [32]
The selected plant material-mature plant of Picrorhiza kurroa Royle ex Benth was collected from Narikkuni, Kerala and was identified on 30/09/2017 by Dr. Shamna, Pharmacologist, MVR Ayurvedic Medical College, Kannur, Kerala, India. 20 gm of the plant material was properly washed with de-ionized water for four times and air dried. The material was the finely cut and mixed with 100 ml of water (deionized) and stirred for 20 min at a temperature of at 60°C, and then boiled. It was then cooled to room temperature and a filtered to 75ml and kept at 40C for further use. Then, 5mL of plant broth was mixed with 45 ml of AgNO3 (0.01M, 99.99%) aqueous solution, and then kept at ambient condition for reaction. The color change of the mixture to dark brown was an indication of AgNP formation. The solution of AgNPs was centrifuged and the excess liquid was evaporated using a dryer to get silver nano-powder with black color. It was stored in a dry place. The integrity and morphology of the AgNPs were analyzed and recorded by standard procedures and protocols including field emission scanning electron microscopy (FESEM with TM-1000, Hitachi, Japan). Also, the silver nanoparticle’s UV-visible absorption was analyzed with a UVVis spectrophotometer (Shimedzu 2400). The particle size was also determined. The FTIR spectroscopy of the green (Picrorhiza kurroa) synthesized silver nanoparticle composite was performed with the equi-grams (100gm) of the product and the spectral grade KBr and pressed under hydraulic pressure. The FTIR spectra were recorded in the 4000-400 cm-1 range.
Selection, collection, and identification of marine invertebrate
Based on the available research reports on the antibacterial potentials of the antimicrobial peptides (AMPs) from marine invertebrates, the source showing remarkable activity was selected. Thus, live specimens of the common squid-Loligo duvauceli Orbigny-was collected from Fishing Harbor, Kozhikode, Kerala, India. The specimen was identified by the scientists at the Calicut Centre CMFRI (Marine Fisheries Research Institute) and confirmed at ZSI (Zoological Survey of India) center, Kozhikode, Kerala.
Extraction and purification of antimicrobial peptides (AMP) from Loligo duvauceli Orbigny [33,34]
20 g of the tissue samples of the marine invertebrate - Loligo duvauceli Orbigny (common squid) was taken and homogenized in 200 ml of 0.2 M sodium acetate, 0.2% Triton X-100, and 1 mM phenyl methyl sulfonyl fluoride which is used as the extraction medium. Then, at 20000Ug, the homogenate was centrifuged for 30 minutes using Himac SCR20BR. After homogenization, the supernatant was collected. Using ion-exchange chromatography, the purification of the crude extract was done, and the fractions were collected separately.
Preparation of AMPs-AgNP conjugates
The anti-microbial peptides-silver nanoparticles were prepared (AMPs-AgNPs) conjugates were prepared mixing them in the ratio 1:1 (100 mg of AMPs was mixed in 1 ml of sterile distilled water and 100mg AgNPs was added drop wise. It was stored for further analysis.
Isolation of bacterial species from common wounds [35]
The clinical samples were collected from different types of wounds and various wound sites of in-patients in the trauma care and the burn unit at the Government hospital, Erode, reported between June and December 2017. The samples were processed, cultured and the isolates were identified using standard procedures and protocols.
Activity of AMPs-AgNPs conjugates against selected wound bacterial isolates
The activity of the AMPs-AgNPs conjugates was tested against ESKAPE members of the predominant isolates from the wounds using standard protocol. Sterile Nutrient Agar media (Composition g/L: Peptone: 5; Beef extract: 3, Sodium chloride: 5, Yeast extract: 5, Agar 15 and final pH adjusted to (7.0 ± 0.2) were prepared and the plates were solidified. Using a pre-calibrated sterile inoculation loop, 0.1% inoculum suspension, from each of the test bacterium was uniformly swabbed on the agar surface. Wells with a diameter of 6mm were prepared on the agar surface using sterile procedures. These well were loaded with the conjugates at a volume of 50μl each and the plates were incubated at 370C for 24–48 hours. The plates were observed for the formation of inhibition zones and were measured and recorded in millimeter.
Anti-ESKAPE activity analysis wound healing spacer materials coated with AMP-AgNP conjugates
The wound healing spacer materials were coated (by a standard dip-coating technique) with the conjugates. The coated spacer materials were cut into discs (20mm) and anti-bacterial activity was observed using disc diffusion method. The anti-ESKAPE testing was done by using the standard AATCC – 147 test methods (Parallel streak method) with the basic principle described elsewhere in this work.
Results
Analysis of the green synthesized AgNPs
The green synthesized AgNPs were topographically analyzed for their structural integrity, particle size and stability by FESEM, UV-Vis Spectrum (absorption peak at 278 nm. and the FT-IR which showed the FT-IR response as shown (Figure 1).
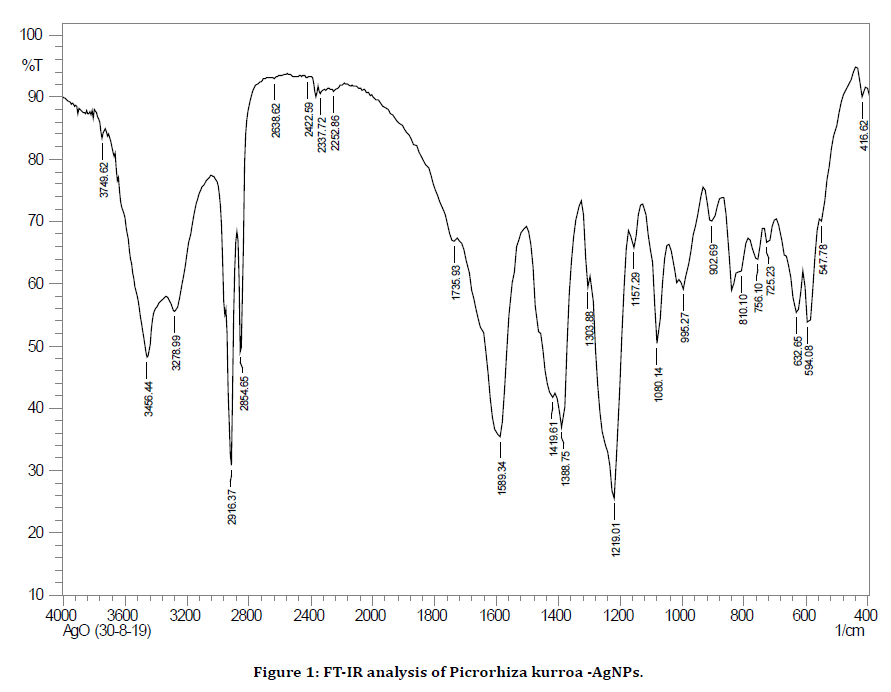
Figure 1. FT-IR analysis of Picrorhiza kurroa -AgNPs.
Distribution of predominant bacterial pathogens in wound isolates
From the total of 83 wound swab samples collected, the identified bacterial pathogens were classified as Gram negative and Gram positive. The predominant ones were taken as they were the members of most antimicrobial resistant (AMR) ESKAPE species. Their distribution was as follows: Gram positive species of Staphylococcus aureus (80.2%), Enterococcus spp. (9.9%) and others the remaining (9.9%). Among the Gram-negative species, Escherichia coli showed the highest presence (29%) followed by Pseudomonas aeruginosa (22%) and Klebsiella pneumonia (19.7%), Acinetobacter baumannii 8.2% and others with an occurrence of 21.1%. Around 62.5% of the total bacterial isolates were, virtually, the members of ESKAPE pathogens and the list of the bacteria from different wounds have been shown in Table 1 and the graphical representation of the percentage of individual ESKAPE member in Gram positive and Gramnegative groups have been shown in Figures 2 and 3.
| Accident/trauma | Diabetic wound/ulcers | Burn wounds |
|---|---|---|
| S. aureus | S. aureus | S. aureus |
| E. faecium | E. faecium | S. epidermidis |
| P. aeruginosa | S. pyogenes | P. aeruginosa |
| E. coli | S. mutants | E. coli |
| K. pneumonia | P. mirabilis | K. pneumonia |
| S. viridans | E. coli | P. mirabilis |
| P. mirabilis | K. pneumonia | Acinetobactor sp. |
| Acinetobactor sp. | S. epidermidis | E. faecium |
Table 1: Distribution of bacterial species in common wounds (the bacteria shown in gray shade are ESKAPE members).
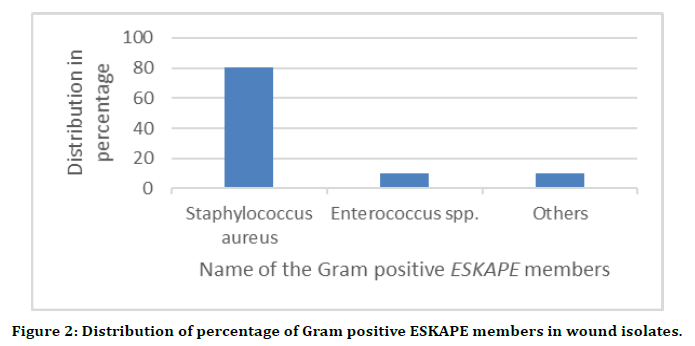
Figure 2. Distribution of percentage of Gram positive ESKAPE members in wound isolates.
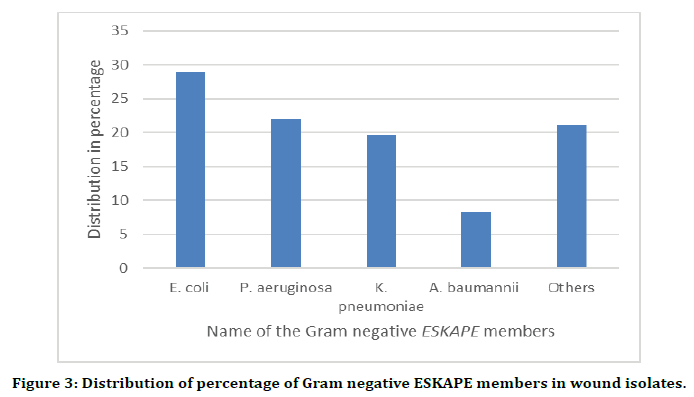
Figure 3. Distribution of percentage of Gram negative ESKAPE members in wound isolates.
Anti-ESKAPE activity of AMP-AgNP conjugates
The AMPs-AgNPs conjugates shown enhanced activity against all the ESKAPE pathogens. The zones of inhibition formed was measured as 22mm ± 0.0 mm against Enterococcus faecium, 23mm ± 1.15 mm against Staphylococcus aureus, 22mm ± 1.15 mm against Klebsiella pneumoniae, 21mm ± 0.0 mm against Acinetobacter baumannii, 21mm ± 1.15 mm against Pseudomonas aeruginosa and 24mm ± 0.0 mm against Escherichia coli. The highest inhibitory zones were obtained against E. coli. Thus, the order of organisms based on inhibitory zones was found to be E. coli.>Staphylococcus aureus>Enterococcus faecium=K. Pneumonia>P. aeruginosa=A. baumanii. The results have been shown in Table 2. Results showing a comparative analysis have also been shown in Figure 4.
| Sl. No. | Test bacteria | Zone of inhibition (in mm) |
|---|---|---|
| 1 | Enterococcus faecium | 22 ± 0.0 |
| 2 | Staphylococcus aureus | 23 ± 1.15 |
| 3 | Klebsiella pneumoniae | 22 ± 1.15 |
| 4 | Acinetobacter baumannii | 21 ± 0.0 |
| 5 | Pseudomonas aeruginosa | 21 ± 1.15 |
| 6 | Escherichia coli | 24 ± 0.0 |
Table 2: Antibacterial activity of conjugates (AMPs-AgNPs) against ESKAPE pathogens.
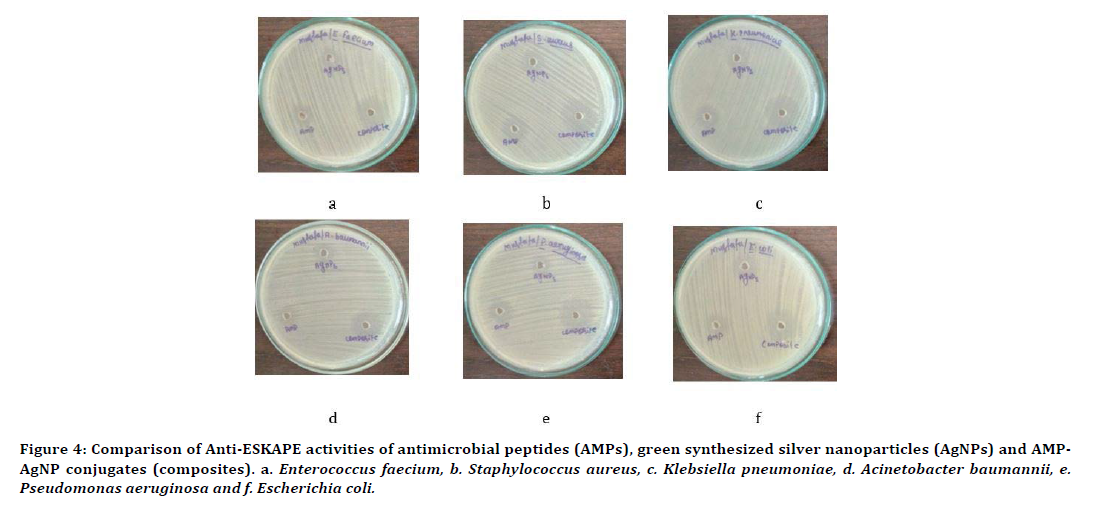
Figure 4. Comparison of Anti-ESKAPE activities of antimicrobial peptides (AMPs), green synthesized silver nanoparticles (AgNPs) and AMPAgNP conjugates (composites). a. Enterococcus faecium, b. Staphylococcus aureus, c. Klebsiella pneumoniae, d. Acinetobacter baumannii, e. Pseudomonas aeruginosa and f. Escherichia coli.
Anti-ESKAPE activity of wound healing spacer materials coated with AMPs-AgNPs conjugates
The growth of all the members of ESKAPE pathogens have been suppressed to a greater extend when they were treated with the wound healing spacer materials coated with AMPs-AgNPs conjugates. E. coli has shown the maximum growth suppression with a 35 mm wide zone whereas A. baumannii formed the lowest width with a length of 30 mm (Table 3 and Figure 5). The comparison of anti-ESKAPE activities of AMPs-AgNPs conjugate and wound healing spacer materials coated with AgNP–AMP conjugates is shown in Figure 6.
| Sl. No. | Test bacteria | Av. width of zone of inhibition (in mm) |
|---|---|---|
| 1 | E. faecium | 32 |
| 2 | S. aureus | 33 |
| 3 | K. pneumoniae | 31 |
| 4 | A. baumannii | 30 |
| 5 | P. aeruginosa | 32 |
| 6 | E. coli | 35 |
Table 3: The average width of the zone of inhibition around the test specimen when screened with wound healing spacer materials coated with AMP-AgNP conjugates.
Figure 5. Anti-ESKAPE activity of wound healing spacer material coated with AgNP–AMP conjugates. a. Enterococcus faecium, b. Staphylococcus aureus, c. Klebsiella pneumoniae, d. Acinetobacter baumannii, e. Pseudomonas aeruginosa and f. Escherichia coli.
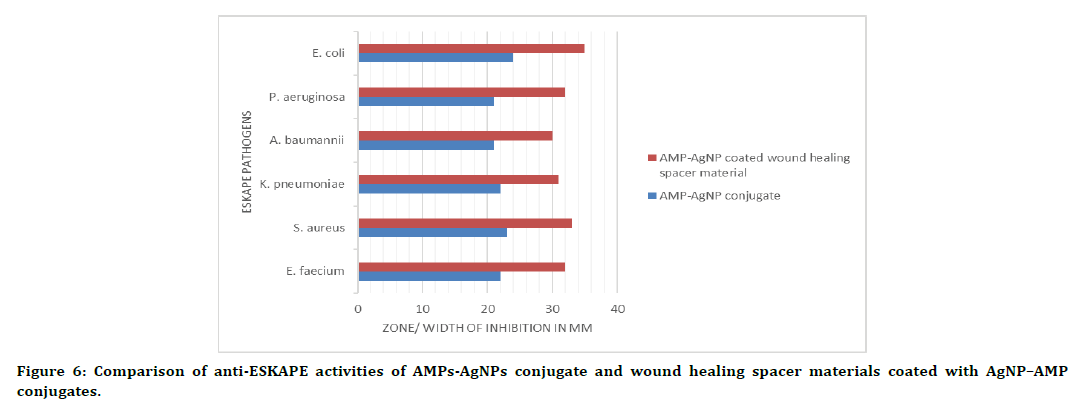
Figure 6. Comparison of anti-ESKAPE activities of AMPs-AgNPs conjugate and wound healing spacer materials coated with AgNP–AMP conjugates.
Discussion
In this study, basically, the antibacterial activities of the wound healing spacer material coated with silver nanoparticles–antimicrobial peptide (AgNP-AMP) conjugates against wound derived ESKAPE pathogens were analyzed. 62.5% of total bacterial species from different types of wound isolated in this study were, virtually, the members of ESKAPE pathogens. Table 1. among the Grampositive species, Staphylococcus aureus showed the highest number with a percentage of 80.2% and in Gram negative pathogens, Escherichia coli was the bacterium with highest occurrence with a presence in 29% of the samples, as described by the Figures 2 and 3. Different studies conducted in various parts of the world underline these findings. An investigation by Shimekaw et al. [6] showed gram-positive Staphylococcus aureus as the most predominant species. Among the gram-negative bacterial bacteria, Pseudomonas aeruginosa was the most predominant 27.1%) followed by Escherichia coli (26.2%). Another investigation showed the Staphylococcus aureus at a rate of 32.1% of isolates and Pseudomonas aeruginosa with a presence of 15.4% of total samples [36].
The AgNPs used in this study was prepared using the medicinal plant Picrorhiza kurroa Royle ex Benth. The green synthesis has many advantages including eco-friendly, cost effective, accurate and the process is simple [16,37]. Also, the plant in this study has been widely studies for its medicinal and antibacterial activities [38]. The green synthesized silver nanoparticles used in the present study has shown activity against all the members of the ESKAPE pathogens as shown in the Figure 4. There are many other investigations reported in which the green synthesized silver nanoparticles shown potential antibacterial activities. For example, silver nanoparticles synthesized using the leaf extract of Moringa oleifera shown activity against eleven pathogens [39]. Another study in which the green synthesis was performed using peels of Citrus sinensis, Citrus limon and Citrus tangerine displayed activities against two strains of the major ESKAPE members i.e., Escherichia coli and Staphylococcus aureus. [40]. In other research, AgNPs synthesized by using extracts of apricot have demonstrated activities against different strains of Staphylococcus aureus, B. cereus, P. aeruginosa and E. coli [41].
The antimicrobial peptide used in this study was extracted from the marine invertebrate-Loligo duvauceli Orbigny (common squid). The AMP has also displayed potential activities against all the ESKAPE pathogens as visible in the Figure 4. This result is in correlation with the findings of many other studies including those described by Mukhopadhyay et al. 2020 [42]. The AgNPAMP conjugate displayed enhanced activity against all the ESKAPE members as shown in Table 2, Figure 4. The highest activity was recorded against E. coli and the lowest against A. baumannii. The antibacterial potentials of silver nanoparticles in conjugation with peptides/ proteins have been studied extensively. The findings of such investigations justify the result in the present study. An analysis by Yang et al. [43] with the silver-containing spacer material had shown 100% reduction of wound bacteria in just one hour. The activity in this work was extended to both Gram-positive members like Staphylococcus aureus and the Gram-negative pathogens like Klebsiella pneumoniae. A work conducted by Pal et al. [44] found that the AgNPAMP conjugates has showed increased activity against Klebisella pneumoniae and E. coli. In another interesting study, the silver nanoparticles coated with multifunctional peptide (MFP@ AgNPs) shown greater activity against multidrug resistant Acinetobacter baumannii [45]. The wound healing spacer material when coated with AgNP-AMP conjugate has shown anti- ESKAPE activities as shown in Table 3 and Figures 6. Various researchers have adapted different methods to enhance the healing and antibacterial activities of wound healing spacer materials. A study by Yingnan Zhu et al. [46]. developed a new material to be used in wound dressings. It was a polycarboxybetaine (PCB) hydrogel with silver nanoparticles (AgNPs) and they displayed activity against Staphylococcus aureus and Escherichia coli. Another experiment by Siju Liu et al. [47] with PEG hydrogel wound dressings have also demonstrated enhanced antibacterial activities against wound pathogens.
Conclusion
The health and economic burdens by the different types of acute and chronic wounds on developing countries are increasing. The wound management is a complex issue as the healing itself is a multi-phased process with a high risk of contamination by pathogens from the environment and from the adjacent tissues. Scientists across the world have been working on this issue and when the microbial resistance against antibiotics increased, their attention turned to different metals–which were used in the past, their nanoparticles, and natural compounds. Hundreds of reports have been published on these investigations and the present study also underlined the previous findings. Green synthesized silver nanoparticles and antimicrobial peptides extract4d from marine invertebrates were shown to have promising activities against “ESKAPE” members of the clinical isolates. When conjugated, AgNPAMP complex showed enhanced activity and the wound healing spacer material when coated with the AgNP-AMP.
Conjugate displayed a higher suppression on all the members analyzed in the order of E. coli.>Staphylococcus aureus>Enterococcus faecium=P. aeruginosa K.>Pneumonia>A. baumanii. The findings in this study underlines the importance for further investigations on silver nanoparticles and their coating on wound healing spacer materials in conjugation with proteinaceous compounds.
Acknowledgement
The authors are thankful to the staff at the Department of Microbiology, Vivekanandha College of Arts and Sciences for Women, Namakkal, Tamil Nadu, India, for their kind cooperation.
Conflict of Interest
Authors declare no conflict of interest.
References
- Homaeigohar S, Boccaccini AR. Antibacterial biohybrid nanofibers for wound dressings. Acta Biomaterialia 2020; 107:25–49.
- Paladini F, Pollini M. Antimicrobial silver nanoparticles for wound healing application: Progress and future trends. Materials 2019; 12:1-16.
- Wang PH, Huang BS, Horng HC, et al. Wound healing. J Chin Med Assoc 2018; 81:94–101.
- Alam MM, Islam MN, Hawlader MD, et al. Prevalence of multidrug resistance bacterial isolates from infected wound patients in Dhaka, Bangladesh: A cross-sectional study. Int J Surg Open 2021; 28:56-62.
- Gibson MK, Forsberg KJ, Dantas G. Improved annotation of antibiotic resistance determinants reveals microbial resistomes cluster by ecology. ISME J 2015; 9:207e16.
- Shimekaw M, Tigabu A, Tessema B. Bacterial Profile, antimicrobial susceptibility pattern, and associated risk factors among patients with wound infections at debre markos referral hospital, Northwest, Ethiopia. Int J Low Extrem Wounds 2020; 1:1-11.
- Bowler PG, Armstrong G. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev 2001; 14:244–269.
- Ohalete Cnork EM. Bacteriology of different wound infection and their antimicrobial susceptibility patterns in Imo state Nigeria. World J Pharm Pharm Sci 2012; 3:1155-1172.
- Bessa LJ, Fazii P, Di Giulio M, et al. Bacterial isolates from infected wounds and their antibiotic susceptibility pattern: some remarks about wound infection. Int Wound J 2015; 12:47-52.
- Duerden BI. Virulence factors in anaerobes. Clin Infect Dis 1994; 18:S253–S259.
- Agwunglefah F, Nwabunike C, Nwaju P. Antibiotic susceptibility pattern of bacteria isolated from surgical wounds of patients attending federal medical center and Christiana Specialist Hospital, Owerri. J Nat Sci Res 2014; 4:2224.
- Bitew A, Tessema A, lema T. Antimicrobial susceptibility pattern of bacterial isolates from wound infections at all Africa leprosy, tuberculosis and rehabilitation training center, Addis Ababa, Ethiopia. EC Microbiol 2018; 14:391-399.
- Mulu W, Abera B, Yimer M, et al. Bacterial agents and antibiotic resistance profiles of infections from different sites that occurred among patients at Debre Markos Referral Hospital, Ethiopia: A cross-sectional study. BMC Res Notes 2017; 10:1-9.
- Bankar N, Wankhade A, Bramhane R, et al. Bacteriological profile of pus/wound swab and antimicrobial susceptibility of staphylococcus aureus isolated from pus & wound swab of indoor patients of tertiary care hospital in durg, Chhattisgarh India. Int J Res Med Sci.2018; 3:1976-1980.
- Mulani MS, Kamble EE, Kumkar SN, et al. Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: A review. Frontiers Microbiol 2019; 10:539.
- Musthafa M, Gobianand K, Manohar M. Anti-eskape activity of green synthesized silver nanoparticles from Picrorhiza kurroa royle ex benth. Int J Pharm Sci Res 2020; 11:5004-5009.
- Lob SH. In-vitro activity of imipenem-relebactam against Gram-negative ESKAPE pathogens isolated by clinical laboratories in the United Statesin 2015. Antimicrob Agents Chemother 2017; 61:2209-2216.
- Beyth N, Houri-Haddad Y, Domb A, et al. Alternative antimicrobial approach: Nano-antimicrobial materials. Evid Based Compl Alt 2015; 2015:246012.
- Rajendran NK, Kumar SS, Houreld NN, et al. A review on nanoparticle-based treatment for wound healing. J Drug Deliv Sci Technol 2018; 44:421–430.
- Liao C, Li Y, Tjong SC. Bactericidal and cytotoxic properties of silver nanoparticles. Int J Mol Sci 2019; 20:449.
- Stoica AE, Chircov C, Grumezescu AM. Nanomaterials for wound dressings: An up-to-date overview. Molecules 2020; 25:1-25.
- Marian B, Alina B. Antimicrobial peptides-natural antibiotics. Rom Biotechnol Lett 2011; 16:6135-6145.
- Alsharif MHK, Alfaki MA, Suliman M, et al. Anatomical abnormalities caused by induced infections in femur and tibia of rattus norvegicus and the efficacy of marine-derived antimicrobial peptides (AMPs) in treatment. J Res Med Dent Sci 2021; 9:141-147.
- Migoń D, Jaśkiewicz M, Neubauer D, et al. Alanine scanning studies of the antimicrobial peptide aurein 1.2. Probiotics Antimicrob Proteins 2019; 11:1042-1054.
- Poyil MM, Bari MN, Prasad SS, et al. Extraction of antimicrobial peptides (AMP) from portunus sanguinolentus herbst, perna viridis linneaus and octopus indicus orbigny: Identifying the best solvent for AMP recovery and determining its anti ESKAPE activity. J Res Med Dent Sci 2021; 9:20-26.
- Tincu JA, Taylor SW. Antimicrobial peptides from marine invertebrates. Antimicrob Agents Chemotherap 2004; 48:3645-54.
- Kumar P, Kizhakkedathu JN, Straus SK. Antimicrobial peptides: Diversity, mechanism of action and strategies to improve the activity and biocompatibility in vivo. Biomol 2018; 8:4.
- Franci G, Falanga A, Galdiero S, et al. Silver nanoparticles as potential antibacterial agents. Molecules 2015; 20:8856-8874.
- Makowski M, Silva ÍC, Pais do Amaral C, et al. Advances in lipid and metal nanoparticles for antimicrobial peptide delivery. Pharmaceutics 2019; 11:588.
- Ramesh S, Grijalva M, Debut A, et al. Peptides conjugated to silver nanoparticles in biomedicine–a “value-added” phenomenon. Biomaterials Sci 2016; 4:1713-1725.
- Manohar M, Musthafa M, Gobianand K. Silver nanoparticle conjugated marine invertebrate antimicrobial peptides (AgNPs-Amps) against gram-negative ESKAPE pathogens. Int J Res Analytical Rev 2019; 6:2348-1269.
- Khan MZ, Tareq FK, Hossen MA, et al. Green synthesis and characterization of silver nanoparticles using Coriandrum sativum leaf extract. J Eng Sci Technol 2018; 13:158-166.
- Khan AN SG. Extraction and screening of bioactive compounds with antimicrobial properties from selected species of mollusk and crustacean. J Clin Cell Immunol 2014; 5:1-5.
- Pushpanathan K, Es B, Sasidharan RS. Characterization of a bioactive protein with antimicrobial property from characterization of a bioactive protein with antimicrobial property from loligo sp. Fishery Technol 2014; 51.
- Robert C Jerris. Practical medical microbiology for clinicians. Wiley-Blackwell, 2015.
- Nahid MA. A longitudinal evaluation of the bacterial pathogens colonizing chronic non-healing wound sites at a United States military treatment facility in the pacific region. Infect Drug Resist 2021; 14:1-10.
- Zarei Z, Razmjoue D, Karimi J. Green synthesis of silver nanoparticles from caralluma tuberculata extract and its antibacterial activity. J Inorg Organomet Polym 2020; 30:4606–4614.
- Firdous J. Evaluation of anti-microbial activity in Picrorhiza kurroa plant extracts using thin-layer chromatography and FTIR. Int J Pharm Technol 2016; 8:15717-1522.
- Islam A, Mandal C, Habib A. Antibacterial potential of synthesized silver nanoparticles from leaf extract of Moringa oleifera. J Adv Biotechnol Exp Ther 2021; 4:67-73.
- Niluxsshun MC, Masilamani K, Mathiventhan U. Green synthesis of silver nanoparticles from the extracts of fruit peel of citrus tangerina, citrus sinensis, and citrus limon for antibacterial activities. Bioinorganic Chem Applica 2021; 2021.
- Vasyliev G, Vorobyova V, Skiba M, et al. Green synthesis of silver nanoparticles using waste products (apricot and black currant pomace) aqueous extracts and their characterization. Adv Materials Sci Eng 2020; 2020.
- Mukhopadhyay S, Bharath Prasad AS, Mehta CH, et al. Antimicrobial peptide polymers: No escape to ESKAPE pathogens-A review. World J Microbiol Biotechnol 2020; 36:131.
- Yang et al. A novel silver-containing absorbent wound dressing based on spacer fabric. 2017, J. Mater. Chem. B. 5, 6786-6793. https://doi.org/10.1039/C7TB01286A
- Pal I, Bechtold T, Redl B, et al. A peptide-nanoparticle system with improved efficacy against multidrug resistant bacteria. Sci Rep 2019; 9:448.
- Li W, Li Y, Sun P, et al. Antimicrobial peptide-modified silver nanoparticles for enhancing the antibacterial efficacy. RSC Advances 2020; 10:38746-38754.
- Zhu Y, Zhang J, Song J, et al. One-step synthesis of an antibacterial and pro-healing wound dressing that can treat wound infections. J Materials Chem 2017; 5:8451-8458.
- Siju Liu, Tao Jiang, Renqi Guo, et al. Injectable and degradable PEG hydrogel with antibacterial performance for promoting wound healing. Am Chem Society 2021; 4:2769-2780.
Author Info
Kuppannan Gobianand1, Muhammad Musthafa1* and Manohar M2
1Department of Microbiology, Vivekanandha College of Arts and Sciences for Women, Namakkal, Tamil Nadu, India2Department of Microbiology, Sadakkathullah Appa College, Tirunelveli, Tamil Nadu, India
Citation: Kuppannan Gobianand, Muhammad Musthafa, Manohar M, Efficacy of Oregano Essential Oil Mouthwash in Reducing Oral Halitosis: A Randomized, Double-Blind Clinical Trial, J Res Med Dent Sci, 2021, 9 (4):501-510.
Received: 29-Mar-2021 Accepted: 14-Apr-2021
