Research - (2021) Volume 9, Issue 3
Antibacterial Activity of Bioactive Glass 45S5 and Chitosan Incorporated as Fillers into Gutta Percha
Ahmed I AL-Jobory* and Raghad AL-Hashimi
*Correspondence: Ahmed I AL-Jobory, Department of Aesthetic and Restorative Dentistry, College of Dentistry, University of Baghdad, Baghdad, Iraq, Email:
Abstract
Aim: To evaluate the anti-bacterial activity of bioactive bioglass 45S5 and Chitosan incorporated as fillers in gutta percha against Enterococcus faecalis.
Methods: The anti-bacterial activity of bioactive bioglass 45S5 and Chitosan incorporated as fillers in gutta percha against Enterococcus faecalis were investigated by measuring the inhibition zone that represent the sensitivity or toxicity of materials against microorganism.
Results: there is highly significant difference between control and new modified gutta percha at all specimens, while the control group showed no sensitivity to microorganism.
Conclusion: Bioactive bioglass BG45S5 and chitosan showed in vitro highly antibacterial effects when mixing with gutta percha against Enterococcus faecalis comparing to commercial (control) gutta percha.
Keywords
Gutta percha, Bioactive bioglass BG45S5, Chitosan, Anti-bacterial, Enterococcus faecalis, Inhibition zone
Introduction
The principal objectives of endodontic therapy are to clean and shape the root canal system and to fill the canal system completely in three dimensions. This aimed to prevent penetration of bacteria and their products into the periapical tissues and develop a hermetic seal [1]. Inadequate obturation of root canal system results in failure of endodontic therapy [2]. A better understanding of root canal anatomy, and improved materials, root canal therapy is achieving an increasingly high overall success rate [3].
However, bacteria inside the root canal system have a significant impact on this success rate. When a tooth is infected before treatment, the success of root canal therapy drops to 79%, as compared to the 93% success rate of root canal treated teeth without apical periodontitis [4]. A few bacterial species, predominantly facultative anaerobes [5,6], are responsible for causing apical periodontitis observed in root canal failures [7]. Root canal failures result either from these microorganisms leaking into the canal after its obturation or from bacteria not eliminated during therapy. The most common genera found to be responsible for root canal failures are Enterococcus, Fusobacterium, Propionibacterium, and Actinomyces [8]. Removing all bacteria in the canal before obturation has proven to be difficult. Therefore, improving the cleaning and disinfection phase of treatment is of crucial importance and has been the impetus for the advancement of instrumentation and irrigation [9,10].
A limited number of studies in the literature have explored the advantages of using obturation materials with antimicrobial properties to prevent bacterial contamination after endodontic procedures [11-13]. Standard gutta-percha has been shown to have an intrinsic effect on very few bacteria species [11,14].
Research has also been con-ducted on the antibacterial activity of gutta-percha points containing established root canal medications, including calcium hydroxide, zinc oxide, chlorhexidine, and iodine-polyvinylpyrrolidone alone or used in combination. However, none of the gutta percha tested possessed an inhibitory activity strong or broad enough to disinfect root canals contaminated with common endodontic pathogens, including E. faecalis [12,14]. Chitosan similar in structure to cellulose composed of one monomer of glucose [15]. It is a polysaccharide comprising copolymers of glucosamine and N-acetyl glucosamine. It can be derived by partial deacetylation of chitin from crustacean shells [16]. It is an environmentally friendly polymer [17]. Chitosan is soluble in diluted acid, in addition, chitosan is stabilized by H– bond network in the solid, providing good mechanical properties [18]. Chitosan has a wide variety of applications such as microbial biosorption or metal removal for wastewater treatment [19].
Chitosan used in different biomedical application due to its antibacterial effect, biocompatibility, and biodegradability. It is used in surgery in tissue engineering which concerned about the restoration of damaged organs using spontaneity scaffolds regeneration that mimic the native tissue [20]. Also, it is used in drug delivery and encapsulation of sensitive drugs, due to it’s an interesting biodegradability, antibacterial activity and antifungal properties [21]. In addition, due to its renewability and bioavailability, since it is the second most abundant polymer in nature after cellulose, Chitosan is applicable in food packaging [22]. Bioactive glasses (BAG) are one such group of biomaterials which are used in the fields of dentistry and orthopaedics to repair or replace damaged bone [23]. A material is said to be bioactive, if it gives an appropriate biological response and results in the formation of a bond between the material and the tissue [24]. Bioactive glasses are composed of calcium and phosphate which are proportionally similar to the hydroxyapatite present within the bone [25]. They also have the unique ability to dissolve in biological fluids and release ions such as silica, sodium and calcium. This ionic dissolution facilitates hydroxyapatite formation and direct bonding to bone and soft tissues [26]. In addition, the quick dissolution with rapid change in pH of the surrounding medium enables these glasses to exhibit anti-bacterial properties [27].
They have a wide range of medical and dental applications and are currently used as bone grafts [24], scaffolds [28] and coating material for dental implants [24].
Methodology
Fabrication of new bioactive gutta percha
The total amount of the Gutta Percha (Dentsply Maillefer, Switzerland) was weighed by four digits sensitive balance ADAM AFA-210LC (UK) which was 1.5 gm. The filer weights were 1% for bioactive powder 45S5 (MO-SCI, USA) and chitosan (SHAANXI SANGHERB BIO-TECH INC, China) where they incorporated by replacement of 0.015 gms (for each filer) of the Gutta- Percha with same weights of powder. So, these percentages were clinically applicable (ISO 6876/2012 standards).
Fabrication of gutta percha
Gutta percha points (1.5 gms) were taken and placed in glass beaker. The beaker has been placed in electrical oven CARBOLITE (UK) at 200ºC for 15min. the beaker has been taken out of the oven, and then the gutta percha points became semi-soft. A small chloroform (Riedelde Haën, German) amount (5ml) has been added to the beaker to solve the gutta percha with continues moving of solvent with glass stick till complete solvent of gutta percha and become like suspension of liquid [29].
Preparing of filers
The bioactive bioglass (with weights 0.015gms represent filler percentage 1%) was dissolved in formic acid (SCR-China) (5ml) and stirring for 3 days until all particles was dissolved. Then the chitosan (with weights 0.015gms represent filler percentage 1%) has been dissolved in 1.0% of the acetic acid (MERCK- German) (v/v) by using magnetic stirrer. The viscous chitosan was adding to previous viscous bioactive bioglass and mix by using a magnetic stirrer. All the viscous mixture of bioactive bioglass and chitosan was adding and mixed together and the total fillers weight was 0.03gm.
Mixing the gutta percha with filers
All the mixture of filler was adding slowly to solvent gutta percha and mixed with a magnetic stirrer till the materials acquired a semi viscous state, it placed in glass petri dishes until complete drying and setting.
Preparing of sample as a disk
A mold was design and fabricated to acquired materials to produce a 5mm width and 2mm height of materials. A 0.035gms of materials was selected and add to cylinder of fabricated devices and a constant scrowing for 1 min was applied to materials to obtain a homogenous disc for all groups (Figures 1-3).

Figure 1: Special mod ready to compress the mixture.

Figure 2: Special mod parts.

Figure 3: Disc of gutta percha (0.035 grms) with 5mm width and 2mm height.
Microbiological study
This study of microbiological was done in the Tikrit University, labs. Of Pharmacy College, to evaluate and test the antibacterial activity (inhibition zone) of the experimental material (New gutta percha) as a disk of 2mm height and 5mm width against selected Enterococcus faecalis microorganism.
Mueller hinton broth (MHB)
Based on the instructions of the manufacturer, the preparation of media was done by placing 21gm of powder in 1 L of the distilled water in graduated beaker. Autoclaving the solution at 1210C at pressure 15psi for 15minutes after the complete dissolution of the powder. Then the solution has been left for cooling at the room temperature and thereafter, tightly closed and kept in a refrigerator until being used (Figure 4).
Figure 4: Preparation of mueller hinton broth (MHB).
Mueller hinton agar (MHA)
Based on the instructions of the manufacturer, the preparation of the media was done by placing 38.0gm of the MHA in 1 L of the distilled water and boiled in a graduated beaker until complete dissolving of the powder. Autoclaving the solution at 121c0 at pressure 15psi for 15min, then left for cooling at 450C-500C. The media has been poured in a pre-sterilized petridish and left to solidify at the room temperature and stored in a refrigerator to the point of usage (Figures 5 and 6) [30,31].
Figure 5: Preparation of mueller hinton agar (MHA).
Figure 6: Pressure up gradually to 121 PSI.
Bacterial culture
Enterococcus faecalis have been maintained in stock cultures that are frozen in brain heart infusion (BHI) broth. E. faecalis colonies have been inoculated in BHI broth at 370C for 24 hours. Enterococcus faecalis were obtain from Media hospital, Erbil, with ATCC 29212 (American Type Culture Collection).
Sensitivity of E. faecalis
Ten experimental materials were used to evaluate the sensitivity of the E.- faecalis to new gutta percha. The media of the MHA in the Petridish has been inoculated with 100.0μl of the E. faecalis suspension which has been prepared as 0.50 Mc-Farland (standard of turbidity 0.50). This number of standards includes about 1x108 of the bacterial cells per ml. The inoculum has been spread in all the directions through the use of the sterilized cotton swaps (Figure 7). Ten petri dishes were prepared, the plates were aerobically incubated for 18h-24h at a temperature of 370c. (Figure 8).

Figure 7: Petri-dishes are ready to inoculated with E. faecalis.

Figure 8: Incubation of plates.
The inhibition zones that are clear zones of no bacterial growth have been measured over the diameter of every one of the petri dished with the use of the digital Vernier caliper, none of the zones have indicated a full bacterial resistance to the agent (Figures 9-11) [32].
Figure 9: Biometra analytic device (BioDocAnalyze).

Figure 10: Biometra analytic device (BioDocAnalyze).
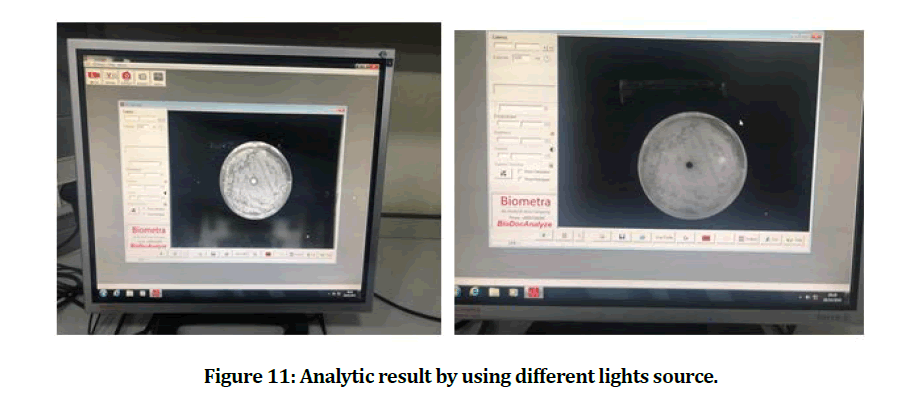
Figure 11: Analytic result by using different lights source.
Results
The mean and standard deviation values of the inhibition zone of the E. faecalis are presented in Table 1. The result showed that the experimental gutta percha that mixed with chitosan and bioactive bioglass has best and power effect against E. faecalis (Figures 12 and 13).
Table 1: Descriptive statistics of inhibition zone.
| Inhibition zone | Experimental gutta percha | Control gutta percha |
|---|---|---|
| 1 | 3.4 cm | 0 |
| 2 | 3.3 cm | 0 |
| 3 | 3.5 cm | 0 |
| 4 | 3.3 cm | 0 |
| 5 | 3.4 cm | 0 |
| 6 | 3.4 cm | 0 |
| 7 | 3.5 cm | 0 |
| 8 | 3.5 cm | 0 |
| 9 | 3.7 cm | 0 |
| 10 | 3.6 cm | 0 |
| Mean | 3.46 cm | 0 |
| SD | 0.21 cm | 0 |
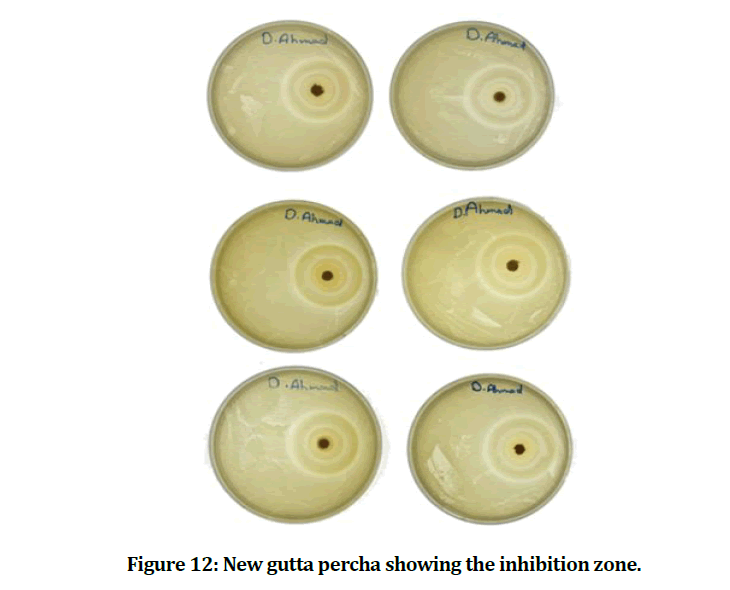
Figure 12: New gutta percha showing the inhibition zone.
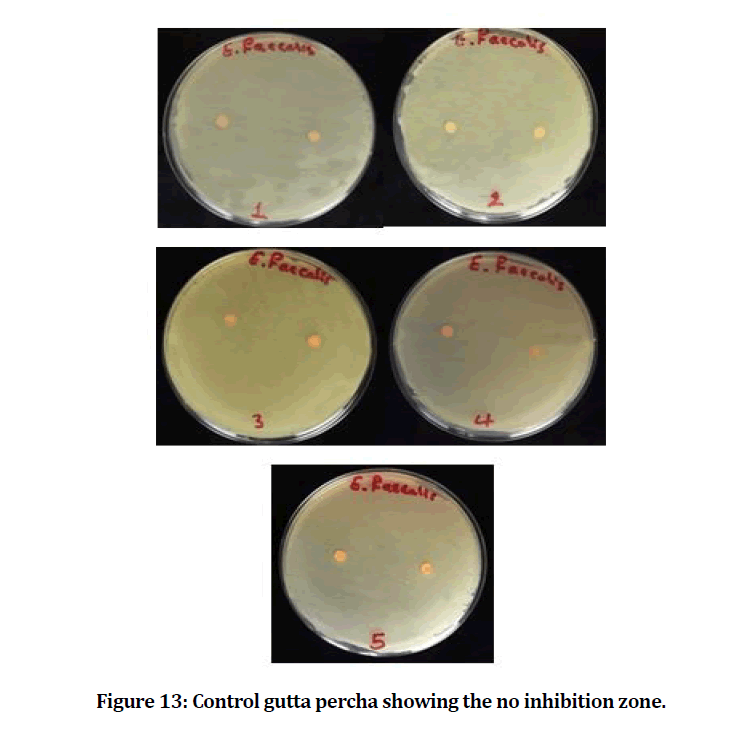
Figure 13: Control gutta percha showing the no inhibition zone.
Discussion
In 1867, Bowman developed gutta percha as one of the significant filling materials, such material contains chlorhexidine and pastes of the calcium hydroxide and, while traditional GP is examined for their anti-bacterial activities via Jhamp et al. [33]. In addition, the results indicated that traditional Gutta percha hadn’t had any antibacterial activities, while the microbial activity is verified via chlorhexidine Gutta percha as well as the Ca (OH)2 pastes. Evidences of small antibacterial activity with regard to the cones of gutta-percha is existing and it is because of zinc oxide (ZnO), the main cones’ component [34]. The major factors related to endodontic treatment failure were persistent microbes in peri radicular region or root canal system. Dissimilar to the primary endodontic infections, that were polymicrobial and dominated via the Gram negative anaerobic rods, the dominant microorganism which has been included in secondary infections were the facultative anaerobes such as the Enterococcus faecalis, such bacteria group is specified as major micro-organism existing in periapical lesions refractory and chronic apical periodontitis [35]. Long-term survival related to E. faecalis in the obturated root canals which are fundamentally depending upon the type of endodontic sealer as well as microbial gelatinase activities, for instance, the organism’s virulence traits [36].
The dental materials are utilized for the obturation in the treatment of the root canal which is needed to be biocompatible and antibacterial, while the anti-bacterial activity which is related to cones of GP was indicated for being unimportant, such disadvantage making them less effective from the microbial infections following the process and should be enhanced. In addition, the materials of ZnO and Ca(OH)2 were majorly indicated in dental practices as anti-microbials [37]. Bioactive bioglass BG 45S5 and chitosan have been shown as an effective medicament and their antimicrobial activity was proved, yet its action is limited via the short time for interaction [36-39].
In recent years, interesting in the antibacterial properties of bioactive glass was increasing. In our finding results, the experimental gutta percha with a great antibacterial effect than conventional (control) gutta percha, this due the filler effect of bioactive bioglass and chitosan. The antibacterial effect of bioactive bioglass was because of increasing the local pH after exchanging ions of Na with the protons in body fluid. Shifting to high alkaline environment was considered to be stressful for the bacteria, that are responding to changing ultrastructure and morphology, changing the expression pattern related to numerous proteins and genes. This may suggest to be the golden mechanism of bioactive glass to mediating the antibacterial action, this result found to be agreed with [40-44].
Another factor associated to the antimicrobial properties related to bioactive glass was the release of the ions of the calcium, silica and phosphate, resulting in perturbations regarding the bacteria’s membrane potential and determine high osmotic pressure. The solutes’ concentration in bacterial cytoplasm was typically high compared to that identified in surrounding environment, leading to positive pressure on cell membranes. Unexpected increment in the external solutes concentrations resulting in fast water efflux as well as pressure drop over cell membrane, which led to modified cell’s size, cell shape, as well as membrane stress levels. These results are in accordance with the results obtained via [45-47]. The bioactive glass is showing activities against many clinically significant strains of bacteria: Gram negative and Gram positive species, also anaerobic and aerobic species [48-51].
Bioactive glass with many chemical compositions as well as many particle sizes. In particular, the particles’ dimensions are determinant of antimicrobial activities. Actually, the decrease in the particle size and subsequent increment in the surface area might be enhancing bioglass contact with the aqueous environment, therefore augment ions’ diffusion, local pH, and, finally, osmotic pressure, such results are in accordance with the results of Waltimo et al. [52] and Coraça‐ Huber et al. [53]. At the same time, chitosan NPs showed high antibacterial activity in comparison to their parental chitosan material because of the elevated ratio of surface to volume. It was indicated for showing antibacterial properties against the oral bacterial pathogens [54,55].
A lot of researches indicated that chitosan showed antimicrobial activity, yet the main approach isn’t totally clarified [56]. The cationic nature is one of these hypotheses. Penetration of bacterial cell walls by chitosan and inhibit DNA transcription and the same time inhibit mRNA synthesis, these were done by chitosan’s low molecular weight, whereas the high molecular weight chitosan has distinctive impact because it is binding to the negatively-charged components on bacterial cell wall, which change the permeability of the cells and blocking transport into cell [37,57,58]. Gram-negative bacteria affect more from this hypothesis because the cell wall of these bacteria had more negative charge than gram-positive bacteria [37,59].
Molecular weight, environmental pH value as well as the degree of acetylating related to chitosan will affect the binding to bacterial cell wall. In addition, the positive charge in the chitosan polymer will increase low pH which lead to strong binding to bacterial cell wall [59]. Younes et al. [60] in 2014 said that the degree of acetylation of chitosan in lower value and lower pH give better antibacterial activity to chitosan. However, this was very obvious toward Gramnegative bacteria.
There is a close relation between antibacterial activity as well as the hydrophilicity related to cell’s wall, thus the action was specific and showing low toxicity towards the mammalian cells [61]. Another reason which made the chitosan more powerful because of the nanoparticles which used in this study. Antibacterial nanoparticulate were indicated for having high antibacterial activity compared to antibacterial powders. In addition, the charge density and high surface area of nanoparticulate, enabled for achieving high interaction degree with negatively charged surface related to the bacterial cells.
For our results the finding is agree with Mohan et al. [37] who indicated that chitosan impregnated gutta percha points exhibited high (and considerable) antifungal and antimicrobial activities, also enhanced the mechanical properties when put to comparison with commercial gutta percha points and using nano-technology for the dental applications for betterments in the clinical areas.
Conclusion
Bioactive bioglass BG45S5 and chitosan showed in vitro highly antibacterial effects when mixing with gutta percha against Enterococcus faecalis comparing to commercial (control) gutta percha.
References
- Punia SK, Nadig P, Punia V. An in vitro assessment of apical microleakage in root canals obturated with gutta-flow, resilon, thermafil and lateral condensation: A stereomicroscopic study. J Conservative Dent 2011; 14:173.
- Bakhtiar H, Heidari N, Mehrvarzfar P, et al. In vitro comparative study of the microbial leakage of one-step, thermafil and lateral condensation techniques. J Contemporary Dent Practice 2012; 13:27-30.
- Friedman S, Mor C. The success of endodontic therapy healing and functionality. CDA J 2004; 32:493-503.
- Lee WC, Hong ST, Shon W. Reconsideration of treatment protocol on the reduction of Enterococcus faecalis associated with failed root canal treatment. J Korean Academy Conservative Dent 2008; 33:560-569.
- Molander A, Reit C, Dahlen G, et al. Microbiological status of root‐filled teeth with apical periodontitis. Int Endodont J 1998; 31:1-7.
- Sundqvist G, Figdor D, Persson S, et al. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re-treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endodont 1998; 85:86-93.
- Möller ÅJ, Fabricius L, Dahlen G, et al. Influence on periapical tissues of indigenous oral bacteria and necrotic pulp tissue in monkeys. Eur J Oral Sci 1981; 89:475-484.
- Siqueira JF. Aetiology of root canal treatment failure: Why well‐treated teeth can fail. Int Endodont J 2001; 34:1-10.
- Abdullah M, Ng YL, Gulabivala K, et ak. Susceptibilties of two Enterococcus faecalis phenotypes to root canal medications. J Endodont 2005; 31:30-36.
- Torabinejad M, Khademi AA, Babagoli J, et al. A new solution for the removal of the smear layer. J Endodont 2003; 29:170-175.
- Moorer WR, Genet JM. Evidence for antibacterial activity of endodontic gutta-percha cones. Oral Surg Oral Med Oral Pathol 1982; 53:503-507.
- Lui JN, Sae‐Lim V, Song KP, et al. In vitro antimicrobial effect of chlorhexidine‐impregnated gutta percha points on Enterococcus faecalis. Int Endodont J 2004; 37:105-113.
- Çobankara FK, Altinöz HC, Erganiş O, et al. In vitro antibacterial activities of root-canal sealers by using two different methods. J Endodont 2004; 30:57-60.
- Podbielski A, Boeckh C, Haller B. Growth inhibitory activity of gutta-percha points containing root canal medications on common endodontic bacterial pathogens as determined by an optimized quantitative in vitro assay. J Endodont 2000; 26:398-403.
- de Alvarenga ES. Characterization and properties of chitosan. Biotechnol Biopolymers 2011; 91:48-53.
- Ray SD. Potential aspects of chitosan as pharmaceutical excipient. Acta Pol. Pharm, 2011; 68:619-622.
- Barzegari A, Shariatinia Z. Fabrication of chitosan-polyethylene oxide electrospun nanofibrous mats containing green tea extract. Iranian J Chem Eng 2018; 15:65-77.
- Garcia CEG, Martínez FAS, Bossard F, et al. Biomaterials based on electrospun chitosan. Relation between processing conditions and mechanical properties. Polymers 2018; 10:257.
- Mahmood HS, Jawad MK. Antibacterial activity of chitosan/PAN blend prepared at different ratios. In AIP Conference Proceedings 2019; 2190:020078.
- Sivashankari PR, Prabaharan M. Prospects of chitosan-based scaffolds for growth factor release in tissue engineering. Int J Biological Macromolecules 2016; 93:1382-1389.
- Younes I, Rinaudo M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Marine Drugs, 2015; 13:1133-1174.
- Muxika A, Zugasti I, Guerrero P, et al. Applications of chitosan in food packaging. 2017.
- Abbasi Z, Bahrololoom ME, Shariat MH, et al. Bioactive glasses in dentistry: A review. J Dent Biomaterials 2015; 2:1-9.
- Farooq I, Imran Z, Farooq U, et al. Bioactive glass: A material for the future. World J Dent 2012; 3:199-201.
- De Carvalho MF, Fernandes RZD, Andrade AL, et al. Bioactive glass with antimicrobial agents: In vitro evaluation. J Med Med Sci 2014; 5:109-112.
- Xiang Y, Du J. Effect of strontium substitution on the structure of 45S5 bioglasses. Chem Materials 2011; 23:2703-2717.
- Hameed AS, Abass AM, Hamood LJ, et al. Effect of Zinc on antibacterial action of bioactive glass coating for dental implant. Med J Babylon 2015; 12:612-617.
- Jones JR, Gentleman E, Polak J. Bioactive glass scaffolds for bone regeneration. Elements 2007; 3:393-399.
- Khawaja RH, Rizwan M, Rashid S. A comparative analysis of adhesion and bond strength of bioactive obturating materials with root dentin. Pakistan Oral Dent J 2016; 36.
- Rafid J, AL-Huwaizi HF. The antibacterial evaluation of Tea tree, Thymus Vulgaris Essential oil and Nigella Sativa oil as root canal medicaments (Invitro study). Master thesis, Department of conservative dentistry, University of Baghdad. 2015.
- Witedja U, Suwartini T, Prahasti AE, et al. Comparing the effectivities of chitosan citrate and chitosan acetate in eradicating Enterococcus faecalis biofilm. Scientific Dent J 2018; 2:1-7.
- CLSI C. Performance standards for antimicrobial susceptibility testing: 25th informational supplement. CLSI document M100-S25. Clinical and Laboratory Standards Institute 2015.
- Jhamb A, Chaurasia VR. In vitro evaluation of antimicrobial activity of different Gutta-percha points and calcium hydroxide pastes. J Int Society Preventive Community Dent 2014; 4:92.
- Gurgel-Filho ED, Feitosa JA, Teixeira FB, et al. Chemical and X-ray analyses of five brands of dental gutta-percha cone. Int Endodont J 2003; 36:302-307.
- Stuart CH, Schwartz SA, Beeson TJ, et al. Enterococcus faecalis: Its role in root canal treatment failure and current concepts in retreatment. J Endodont 2006; 32:93-98.
- Hage W, De Moor RJ, Hajj D, et al. Impact of different irrigant agitation methods on bacterial elimination from infected root canals. Dent J 2019; 7:64.
- Mohan A, Dipallini S, Lata S, et al. Oxidative stress induced antimicrobial efficacy of chitosan and silver nanoparticles coated gutta-percha for endodontic applications. Materials Today Chem 2020; 17:100299.
- Pazos-Ortiz E, Roque-Ruiz JH, Hinojos-Márquez EA, et al. Dose-dependent antimicrobial activity of silver nanoparticles on polycaprolactone fibers against gram-positive and gram-negative bacteria. J Nanomat 2017; 2017.
- Drago L, Toscano M, Bottagisio M. Recent evidence on bioactive glass antimicrobial and antibiofilm activity: A mini-review. Materials 2018; 11:326.
- Allan I, Newman H, Wilson M. Antibacterial activity of particulate Bioglass® against supra-and subgingival bacteria. Biomaterials 2001; 22:1683-1687.
- Ran S, He Z, Liang J. Survival of Enterococcus faecalis during alkaline stress: Changes in morphology, ultrastructure, physiochemical properties of the cell wall and specific gene transcripts. Archives Oral Biol 2013; 58:1667-1676.
- Begum S, Johnson WE, Worthington T, et al. The influence of pH and fluid dynamics on the antibacterial efficacy of 45S5 bioglass. Biomed Materials 2016; 11:015006.
- Bortolin M, Romanò CL, Bidossi A, et al. BAG-S53P4 as bone graft extender and antimicrobial activity against gentamicin-and vancomycin-resistant bacteria. Future Microbiol 2018; 13:525-533.
- Zhang D, Leppäranta O, Munukka E, et al. Antibacterial effects and dissolution behavior of six bioactive glasses. J Biomed Materials Res 2010; 93:475-483.
- Jones JR. Review of bioactive glass: From Hench to hybrids. Acta Biomater 2013; 9:4457-4486.
- Pilizota T, Shaevitz JW. Plasmolysis and cell shape depend on solute outer-membrane permeability during hyperosmotic shock in E. coli. Biophysical J 2013; 104:2733-2742.
- Salinas AJ, Vallet-Regi M, Heikkilä J. Use of bioactive glasses as bone substitutes in orthopedics and traumatology. In Bioactive Glasses Woodhead Publishing 2018; 337-364.
- Leppäranta O, Vaahtio M, Peltola T, et al. Antibacterial effect of bioactive glasses on clinically important anaerobic bacteria in vitro. J Materials Sci 2008; 19:547-551.
- Romanò CL, Logoluso N, Meani E, et al. A comparative study of the use of bioactive glass S53P4 and antibiotic-loaded calcium-based bone substitutes in the treatment of chronic osteomyelitis: A retrospective comparative study. Bone Joint J 2014; 96:845-850.
- Munukka E, Leppäranta O, Korkeamäki M, et al. Bactericidal effects of bioactive glasses on clinically important aerobic bacteria. J Materials Sci 2008; 19:27-32.
- Drago L, Vecchi ED, Bortolin M, et al. Antimicrobial activity and resistance selection of different bioglass S53P4 formulations against multidrug resistant strains. Future Microbiol 2015; 10:1293-1299.
- Waltimo T, Brunner TJ, Vollenweider M, et al. 2007. Antimicrobial effect of nanometric bioactive glass 45S5. J Dent Res 2007; 86:754-757.
- Coraça‐Huber DC, Fille M, Hausdorfer J, et al. Efficacy of antibacterial bioactive glass S53P4 against S. aureus biofilms grown on titanium discs in vitro. J Orthopaedic Res 2014; 32:175-177.
- Divya K, Vijayan S, George TK, et al. Antimicrobial properties of chitosan nanoparticles: Mode of action and factors affecting activity. Fibers Polymers 2017; 18:221-230.
- Makkar H, Verma SK, Panda PK, et al. Molecular insight to size and dose-dependent cellular toxicity exhibited by a green synthesized bioceramic nanohybrid with macrophages for dental applications. Toxicol Res 2018; 7:959-969.
- Randy CF, Tzi BN, Jack HW. Chitosan: An update on potential biomedical and pharmaceutical application. Mar Drugs 2015; 13:5156-5186.
- Sudarshan NR, Hoover DG, Knorr D. Antibacterial action of chitosan. Food Biotechnol 1992; 6:257-272.
- Zheng LY, Zhu JF. Study on antimicrobial activity of chitosan with different molecular weights. Carbohyd Polym 2003; 54:527–530.
- Rhoades J, Roller S. Antimicrobial actions of degraded and native chitosan against spoilage organisms in laboratory media and foods. Appl Environ Microbiol 2000; 66:80-86.
- Younes I, Sellimi S, Rinaudo M, et al. Influence of acetylation degree and molecular weight of homogeneous chitosans on antibacterial and antifungal activities. Int J Food Microbiol 2014; 185:57-63.
- Kong M, Chen XG, Xing K, et al. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int J Food Microbiol 2010; 144:51–63.
Author Info
Ahmed I AL-Jobory* and Raghad AL-Hashimi
Department of Aesthetic and Restorative Dentistry, College of Dentistry, University of Baghdad, Baghdad, IraqCitation: Ahmed I AL-Jobory, Raghad AL-Hashimi, Antibacterial Activity of Bioactive Glass 45S5 and Chitosan Incorporated as Fillers into Gutta Percha, J Res Med Dent Sci, 2021, 9 (3):108-117.
Received: 16-Feb-2021 Accepted: 11-Mar-2021 Published: 18-Mar-2021
