Research - (2021) Volume 9, Issue 4
Antibacterial Activity of Alcoholic Cinnamon Extract and Chlorhexidine on Streptococcus Mitis as Primary Colonizers in Periodontal Disease
Ahmed Abdulwahid Hasan and Ayser Najah*
*Correspondence: Ayser Najah, Department of Periodontics, College of Dentistry, University of Baghdad, Iraq, Email:
Abstract
Background: Cinnamon is one of the oldest herbs that has been used by humankind for cooking, seasoning, bakery, food preservation, and drinks. Recently, researchers have been focusing on the antibacterial effect of cinnamon, this study is aimed to determine the antibacterial effect of alcoholic cinnamon extract on Streptococcus mitis (S.mitis) in vitro. And the combination of chlorhexidine (CHX) with cinnamon alcoholic extract relationship type. Periodontal diseases are one of the most common diseases affecting humanity. Biofilm is the causative factor for periodontal disease. Biofilm is composed of various microorganisms. Streptococci Mitis is one of the most common early colonizers within dental biofilms, which cause periodontal disease. Reduction of the microorganism found in biofilm leads to a reduction in periodontal diseases, thus antimicrobials agents have been studied as an adjunct for ordinary therapy. Chlorhexidine is the most known agent that has been used as a chemical plaque controlling agent. It has been reported to be the most active agent used in mouthwash. As well as CHX is one of the most important known antiseptics, low concentration is bacteriostatic and on high concentration, it is bactericidal.
Aim/Objectives: To evaluate the antibacterial effect of alcoholic cinnamon extracts on Streptococcus mitis bacteria. To study the combination between Chlorhexidine and alcoholic cinnamon MIC when mixed.
Materials and Methods: The first step of the present study was to prepare the alcoholic cinnamon extract, the process of extraction type was maceration type. The second step of the present study was preparing and isolating Streptococcus mitis. The samples were taken from supra-gingival plaque using cotton swabs of 20 volunteers. Followed by morphological and microscopical examination, in addition to, biochemical tests. Vitek 2 machines were used to ensure the correct identification of Streptococcus mitis. The data were analyzed by using descriptive statistics including mean and standard deviation, LSD was done for the analysis of MIC of (alcoholic cinnamon extracts) against (Streptococcus mitis Bacteria). The significance of all the statistical tests was determined at p<0.05 using SPSS (Statistical Package for social science) version 25 software.
Results: Alcoholic cinnamon extract showed a higher antibacterial effect on Streptococcus mitis than CHX. The combination of the combination between alcoholic cinnamon extract and CHX gave the highest antibacterial effect than each one separated (synergistic).
Conclusion: Cinnamon is a potent antimicrobial agent. It is an effective, safe, and cost affordable material that can be used as an alternative agent for chlorhexidine in oral hygiene products
Keywords
Anti-bacterial, Cinnamon extract, Chlorhexidine, S. mitis
Introduction
Periodontal diseases are one of the most common diseases affecting human beings, the diseases start with bacterial adherence and the development of biofilms on the surfaces of the tooth including either natural or restored [1]. Dental plaque is pale yellowish in color biofilm which forms naturally on the teeth. A dental plaque is formed by colonizing bacteria attempting to attach themselves to the surface of the tooth. Dental biofilm, commonly referred to it as dental plaque, is a mixture of about a thousand bacterial species that constitute part of the sophisticated ecosystems of the mouth [2]. Dental plaque begins when present bacteria attach to teeth and start multiplying. Plaque may build on teeth either in supragingival plaque, or subgingival plaque, or both [3]. The plaque is built of various colonies of microorganisms such as bacteria, yeast stuck together in a gel-like organic material matrix composed of bacterial byproducts comprising sugar, food debris, and body tissue [4]. The dominant initial colonizers of teeth are Gram-positive facultative anaerobic cocci and rods, involving Streptococcus and Actinomyces species. These initial colonizers make a foundation for further development of dental biofilm [5]. Streptococcus mitis, Streptococcus oralis, and Streptococcus sanguinis of the Mitis group streptococci [6,7] are closely correlated to Streptococcus pneumonia, the important human pathogen.
The streptococcus species are part of human normal oral commensal flora, but sometimes, they cause acute or chronic episodes of disease as opportunistic human pathogens, unlike S. pneumonia. They are linked with gingival disease and caries and now and then cause sub-acute infective endocarditis [8]. Because they are considered as the early colonizers in the development of the dental biofilm [9]. They can gain natural competence for hereditary transformation as S. pneumonia [10] and have horizontal transferring of the gene, such as virulent genes, that has been recorded between these species [6,11-13]. The emergence of multidrug resistant (MDR) microorganisms has turned to be a danger to general health [14-16]. This worrisome lead to an immediate urge for the invention of contemporary antibacterial and treatment strategies.
Cinnamon's unique healing abilities come from three basic types of components found in its bark. These oils contain active components called cinnamaldehyde, cinnamyl acetate, and cinnamyl alcohol, plus a wide range of other volatile substances [17]. Cinnamon has been used in food preservatives owing to its antioxidant properties, antibacterial, insecticidal, and nematicidal activities. It has been widely used in the food industry. Also, it is employed in seasonings, sauces, bakery, confectionery, and drinks [18].
Cinnamaldehyde inhibits cell wall biosynthesis and causes cell membrane dysfunction [19]. The presumed mechanism of antimicrobial action of Cinnamon is probably due to the presence of Cinnamaldehyde, an aromatic aldehyde, which has electronegative and interferes in biological processes including electron transfer and reacts with nitrogen having components, for example, proteins and nucleic acids, and therefore stops the growth of the microorganisms, Most of the studies on cinnamon suggest that it is not harmful to humans and may be used as an agent to inhibit the growth of bacteria, fungi, and yeast [20].
Aim/Objectives
To evaluate the antibacterial effect of alcoholic cinnamon extracts on Streptococcus mitis bacteria.
To study the combination between Chlorhexidine and alcoholic cinnamon MIC when mixed.
Materials and Methods
Study design/sample
The material and methods were made through two steps:
The first step was the preparation of the cinnamon extract,
The second step was the preparation of the bacteria (streptococcus mitis)
First, preparation of the cinnamon
The type of cinnamon used in this study was SirLanka type which was bought from a local attar market (Jannat Alashab). The extraction was prepared at the University of Baghdad/ College of Agriculture/Department of medical herbs/University of Baghdad. The extraction process was made using the maceration process. Figures 1 and 2.
Figure 1: Showed cinnamon bark.
Figure 2: Cinnamon powder.
Second, The preparation of bacteria
Bacterial samples were taken from supragingival plaque through cotton swabs. A group of 20 volunteers was volunteered to give a sample then bacterial isolation, culturing, identification, and activation were done.
Procedure
The procedure was done according to the method of Shtayeh and Abu ghadeib.
The bark was ground using a grinding machine (Silvercrest mixer) into a fine powder. Then the powder was weighed using an electronic balance (SF-400) 20 gram of powder was placed in a glass beaker of 500 ml size and then 200 ml 70% ethyl alcohol was added to it, it was then placed in a shaker incubator (Binder BF-720) at 35 °C for 24 hours and it was filtered using Whatman filter paper No.1, then the product was centrifuged at 3000 rpm for 10 minutes, then it was dried in the oven (Oven at 40 °C.), then kept in dark and tight tubes in the freezer at a temperature of -18 °C until use.
Determination of the minimum inhibitory concentration (MIC)
The minimum inhibitory concentration (MIC) was defined as the lowest concentration of any anti-microbial agent that would inhibit the visible growth of the selected micro-organism [21-23]. The determination of the MIC was done by using the broth dilution method, it is one of the most basic antimicrobial susceptibility methods. This procedure involves preparatory two folds dilution of the cinnamon extract used (16 %, 8 %, 4 %, 2%, and 1%) in Muller Hinton broth dispersed in tubes containing a minimum volume of 2 ml, then, each tube is inoculated with microbial inoculum (S. mitis) prepared in the same medium of standardized microbial suspension adjusted to 0.5 McFarland scale. After well mixing, the inoculated tubes are incubated aerobically at 37 C for 24 hours. After 24 hours the tubes were checked by the naked eye. The tube that showed turbidity meant that there was bacterial growth and were excluded, while the tubes that revealed a clear vision were meant as no bacterial growth. The first tube that lacks turbidity was the MIC concentration.
Preparation of bacteria
A cotton swab was taken from supragingival plaque. A group of 20 volunteers with moderate oral hygiene were selected. The samples were put into a plane tube continuing 10 ML of brain heart infusion broth. Isolation of the streptococci is performed through homogenizing plaque samples by a vortex mixer for two minutes, then ten-fold dilution was performed by transferring 0.5 ml of saliva to 4.5 ml of phosphate buffer saline (pH 7.0). From dilution 10-3, 10-4, and 10-5 of collected samples, 0.1 was taken and spread in duplicate on the MSB agar media, the plates were incubated anaerobically using a candle for 48hr. at 37º C then aerobically for 24 hr. at room temperature. The identification of streptococcus mitis was done by morphological and biochemical characteristics, in a sterile condition, the colony was taken from the blood agar plate and exposed to Grams stain.
Grams stains
The single isolated colony mixed with a drop of distal water on a glass slide, then spread, dried, then fixed with heat, after that coated with crystal violet for 1 min, then rinsed with distal water, coated with Lugols iodine for 1 minute then rinsed, decolorized with acetone for 30 seconds and rinsed, finally stained with safranin for 1 minute then rinsed and dried (Figure 3).
Figure 3: Gram-stained bacteria under the microscope.
A blood Hemolysis test used to evaluate the capability of Streptococcus mitis to bleach the heme iron via hydrogen peroxide (H2O2), forming to greenish color on the blood agar [24].
Catalase production test
This test was achieved on colonies of bacteria. Hydrogen peroxide 3% (H2O2) had been used to evaluate the ability to create catalase enzyme by bacteria [25].
Vitek 2 machine
Vitek 2 for identifying the specific bacterial type (Table 1 and Figure 4) [26].
Figure 4: Vitek 2 machine.
Table 1: Streptococcus mitis identification by Vitek 2.
| Identification information | Card: GP | Lot number: 2420322103 | Expires: Nov 8, 2020 13:00 CDT |
|---|---|---|---|
| Status: Final | Analysis time: 5.78 hours | Completed: Nov 8, 2020 16:13 CST | |
| Organism origin | Vitek 2 | ||
| Selected organism | Streptococcus mitis | ||
| 95% probability | |||
| Bio number: 021010364305511 | |||
| Confidence: Particularly good identification | |||
The experiment of the study
Antimicrobial effect of Streptococci mitis, St. sanguinis, and St. Salivarius to different Concentrations of Cinnamon Extracts, Chlorhexidine, and Deionized Water in Vitro.
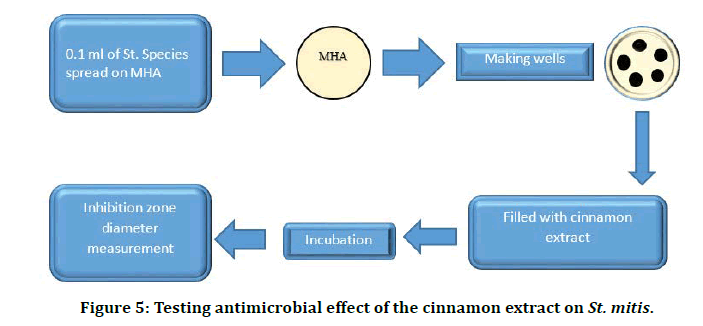
Different concentrations of cinnamon extract and were used in this experiment, the concentrations were as follows: 1.5%, 2.5%, 5%, and 10%. The Agar well technique was used in this experiment to study the sensitivities of bacteria to these agents on Mueller Hinton agar on ten isolates of streptococcus species (Figure 5).
Figure 5: Testing antimicrobial effect of the cinnamon extract on St. mitis.
Procedure
â??A volume of 25 ml of MHA was poured into sterile glass Petri dishes, left at room temperature for 24 hr.
â??To each plate 0.1 ml of streptococci species inoculum was spread, left for 20 minutes at room temperature then wells of equal size and depth were prepared about (three-four wells) in each plate, each well was filled with 0.2ml of the test agents.
â??Plates were left at room temperature for one hour then incubated anaerobically for 24 hr. at 37°C, each zone of inhibition was measured across the diameter of each well. No zone of inhibition indicated complete resistance of bacteria to the test agent.
Test agents
â??99 Cinnamon extracts :( 1.25%, 2.5%, 5% and 10%). â??99 CHX gluconate 0.2%. â??99 DW.
Results
Agar well diffusion method was used to determine the sensitivity of S. mitis to different concentrations of alcoholic cinnamon extracts, CHX (0.2%), and D.W. in vitro. The diameter of the inhibition zone (clear zone which represents no growth of S. sanguinis around each well) was found to increase as we increased the concentration of cinnamon extracts (Figures 6 and 7).
Figure 6: Sensitivities of S. mitis to different concentrations of alcoholic cinnamon extract.
Figure 7: Sensitivities of S. mitis to the combination of the alcoholic cinnamon extract MIC with CHX and CHX alone.
Determination of the minimum inhibitory concentration (MIC)
Determination of the minimum inhibitory concentration (MIC) was done using the Broth dilution method (Figure 8).
Figure 8: MIC of alcoholic cinnamon extract.
The MIC value for S. mitis was
The alcoholic was 2% (0.2 mg/ml)
The chlorhexidine (CHX) showed no turbidity which meant the absence of bacterial cell growth (S. mitis) at 0.2% concentration.
D.W. showed no turbidity which meant the absence of bacterial growth.
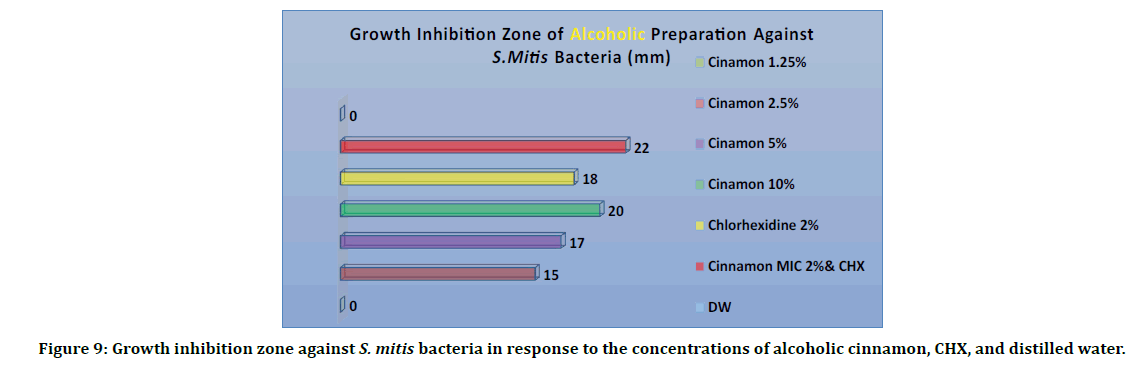
Figure 9 showed the results of alcoholic cinnamon extracts. Alcoholic cinnamon extracts were used by four different concentration (1.25%, 2.5%, 5% and 10 %), CHX (0.2%) as positive and distilled water (D.W). as negative control. The inhibition zone for an alcoholic was measured. The 1.25% cinnamon extract gave, as well as the distilled water, 0 mm. The concentration of 2.5 % gave a 15 mm inhibition zone (clear circle around the well in the agar disk). The concentration of 5% gave us a 17 mm inhibition zone. The concentration of 10% gave us 20 mm. the CHX by itself only gave us 18 mm. The combination of alcoholic cinnamon MIC (2%) and CHX gave us a 22 mm inhibition zone. the results of the different concentrations used in the sensitivity test revealed that the mixture of the alcoholic cinnamon extract and CHX gave the highest inhibition number 22 mm, followed by CHX 20 mm. The mixture of alcoholic cinnamon MIC and CHX showed that the cinnamon had improved the activity of CHX.
Figure 9: Growth inhibition zone against S. mitis bacteria in response to the concentrations of alcoholic cinnamon, CHX, and distilled water.
Table 2 showed the comparison in the mean of growth inhibition zone between different concentrations of cinnamon, CHX, and the combination of cinnamon MIC and CHX using the ANOVA test. This was specifically done against the S. mitis. The result was significantly higher (P<0.05). It was clear that there was a difference in the effect among the different concentrations that were used against S. mitis, the different preparations did not give the same inhibition number, as we mentioned above, the inhibition zone increased as we increased the concentration.
Table 2: Comparison in the mean of growth inhibition zone against S. mitis bacteria between different concentrations and preparations of cinnamon and CHX.
| Concentration and preparation | Growth inhibition zone against S. mitis bacteria (mm) Mean ± SD | F-test | P-Value |
|---|---|---|---|
| Alcoholic | |||
| 2.50% | 15 ± 0.1 | 10.597 | 0.001 |
| 5% | 17 ± 0.13 | ||
| 10% | 20 ± 0.18 | ||
| CHX 2% | 18 ± 0.5 | ||
| CHX 2% + Alcoholic Cinn MIC (1.5%) | 22 ± 0.16 | ||
Post hoc tests or least significant value (LSD) was run to confirm the differences occurred in growth inhibition zone between different preparations and concentrations of cinnamon and CHX 2% and showed that in alcoholic preparation, mean of growth inhibition zone that developed by mixture of cinnamon and 2% CHX was significantly higher (P<0.05) than that in all other concentrations except 10% cinnamon when it was not significantly different (P=0.095). Also, it was increased by increasing the concentration of cinnamon except in concentrations of 2.5% and 5% when it was not significantly different (Table 3).
Table 3: Post hoc tests to confirm the differences occurred in the growth inhibition zone of S. mitis bacteria between different preparations and concentrations of cinnamon and CHX 2%.
| Mean of growth inhibition zone (mm) | Concentration and preparation | P-Value | ||||
|---|---|---|---|---|---|---|
| 2.5 | 5 | 10 | CHX 0.2% | CHX 2%+Cinn 2% | ||
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| Alcoholic | 15 ± 0.1 | 17 ± 0.13 | - | - | - | 0.095 |
| 15 ± 0.1 | - | 20 ± 0.18 | - | - | 0.001 | |
| 15 ± 0.1 | - | - | 18 ± 0.5 | - | 0.014 | |
| 15 ± 0.1 | - | - | - | 22 ± 0.16 | 0.001 | |
| - | 17 ± 0.13 | 20 ± 0.18 | - | - | 0.014 | |
| - | 17 ± 0.13 | - | 18 ± 0.5 | - | 0.339 | |
| - | 17 ± 0.13 | - | - | 22 ± 0.16 | 0.001 | |
| - | - | 20 ± 0.18 | 18 ± 0.5 | - | 0.095 | |
| - | - | 20 ± 0.18 | - | 22 ± 0.16 | 0.095 | |
| - | - | - | 18 ± 0.5 | 22 ± 0.16 | 0.001 | |
Discussion
Cinnamon has been used by humankind for thousands of years [27-29], for cooking, medical purposes for perfumes [30], the unique, sweet, and distinguishable odor made cinnamon one of the luxurious spices that have been used by rich people [31,32].
Contemporary medicine focuses on natural materials that safe for humans and animals, friendly to the environment, and effective against pathogens. Plants and herbs are a natural material that grows on earth without chemical reactions intervention [33].
Cinnamon extracts cause inhibition of bacterial growth in many ways. The action of cinnamon could be due to the predominant active component present in cinnamon which is cinnamaldehyde and cinnamyl acetate which leads to inhibition of proton motive force, the chain of respiration, transfer of the electron, and substrate oxidation, resulting in unbinding of oxidative phosphorylation, active transport inhibition, loss of pool metabolites, and damages to the synthesis of DNA, RNA, proteins, polysaccharides, and lipids [34]. In addition to that, the property of the extract of cinnamon and its components is its hydrophobicity, which enables it to disturb the lipid bilayer of the membrane of the cell, rendering the wall more permeable to protons. Extensive leakage from bacterial cells or the exit of critical molecules and ions ultimately leads to bacterial cell death [35].
Chlorhexidine is the most known agent that has been used as a chemical plaque controlling agent. It has been reported to be the most active agent used in mouthwash. As well as CHX is one of the most important known antiseptics, low concentration is bacteriostatic and on high concentration, it is bactericidal [36]. In addition to that, CHX affects a broad range of bacteria covering gram-positive and gram-negative and even including some fungi [37]. The previous findings have shown that washing with 10 ml of CHX gluconate solution 0.2%, twice a day, almost completely stops the progress of the microbial plaque in humans. Therefore, gingivitis and periodontitis can be prevented, and this is confirmed by empirical studies. Clinical studies that used CHX for several months as a mouthwash showed a 45 to 61% decrease of the microbial plaque and a 27 to 67% decrease in gingivitis [38]. CHX does not interact with any microbial enzymes or receptors and hence does not lead to resistance from organisms [39].
Oral Streptococci of the Mitis group Streptococci [40,41], as S. mitis, S. sanguinis, and S. salivarius, which are mutually related to the S. pneumonia (the significant human pathogen). They are initial colonizers in the development of the biofilms of the oral cavity [42], Streptococcus mitis, Streptococcus sanguinis, and S. salivarius were chosen in the presented study due to their function in the development of plaque and eventually the periodontal disease [43]. In addition to the fact that they are the most found bacteria of dental plaque as primary plaque colonizing bacteria [44].
The explanation for the synergism between the extract of cinnamon and CHX could be because the CHX is a dicationic molecule with has high tendency to attach to an anionic molecule, the cell membrane is anionic where CHX bind to it leading to disruption of the cell wall, the disruption of the bacterial cell wall enhances the entry of cinnamon molecule inside the cell in higher quantity leading to more effective action [45-47]. Once the cinnamon gets inside the bacterial cell, it will cause proton motive force, the chain of respiration, transfer of the electron, and substrate oxidation resulting in unbinding of oxidative phosphorylation, active transport inhibition, loss of pool metabolites, and damages to the synthesis of DNA, RNA, proteins, polysaccharides, and lipids [34].
Conclusion
Cinnamon has a potent antibacterial effect against Streptococcus mitis. The combination between CHX and cinnamon is synergistic. Curcumin can be a promising therapeutic agent as an alternative to CHX.
References
- Newman MG, Takei H, Klokkevold PR, et al. Carranza's clinical periodontology. 13th Edn 2018; 1710-1712.
- Vitt A, Gustafsson A, Ramberg P, et al. Polyhexamethylene guanidine phosphate irrigation as an adjunctive to scaling and root planing in the treatment of chronic periodontitis. Acta Odontologica Scandinavica 2019; 77:290-295.
- Liaw A, Miller C, Nimmo A. Comparing the periodontal tissue response to nonâ?surgical scaling and root planing alone, adjunctive azithromycin, or adjunctive amoxicillin plus metronidazole in generalized chronic moderateâ?toâ?severe periodontitis: A preliminary randomized controlled trial. Australian Dent J 2019; 64:145-52.
- Palombo EA. Traditional medicinal plant extracts and natural products with activity against oral bacteria: potential application in the prevention and treatment of oral diseases. Evidence-Based Complementary Alternative Med 2011; 2011.
- Zandbergen D, Slot DE, Cobb CM, et al. The clinical effect of scaling and root planing and the concomitant administration of systemic amoxicillin and metronidazole: A systematic review. J Periodontol 2013; 84:332-51.
- Buommino E, Scognamiglio M, Donnarumma G, et al. Recent advances in natural product-based anti-biofilm approaches to control infections. Mini Reviews Med Chem 2014; 14:1169-82.
- Pai PG, Dayakar MM, Nath AR, et al. Phytotherapeutics in the management of periodontal disease-A review. J Res Dent Sci 2019; 10:82.
- Lawande SA. Therapeutic applications of turmeric (Curcuma longa) in dentistry: A promising future. J Pharm Biomed Sci 2013; 27:586-591.
- Anuradha BR, Bai YD, Sailaja S, et al. Evaluation of anti-inflammatory effects of curcumin gel as an adjunct to scaling and root planning. J Int Oral Health 2015; 7:90-93.
- Deevaraj SD, Neelakantan P. Curcumin-pharmacological actions and its role in dentistry. Asian J Pharm Res Health Care 2014; 6:19-22.
- Listgarten M. The role of dental plaque in gingivitis and periodontitis. J Clin Periodontol 1988; 15:485-487.
- Akcali A, Huck O, Tenenbaum H, et al. Periodontal diseases and stress: a brief review. J Oral Rehabil 2012; 40:60-68.
- Behal R, Mali AM, Gilda SS, et al. Evaluation of local drug-delivery system containing 2% whole turmeric gel used as an adjunct to scaling and root planing in chronic periodontitis: A clinical and microbiological study. J Indian Soc Periodontol 2011; 15:35-38.
- Bhatia M, Urolagin SS, Pentyala ΚB, et al. Novel therapeutic approach for the treatment of periodontitis by curcumin. J Clin Diagnostic Res 2014; 8:65-69.
- Gottumukkala SN, Koneru S, Mannem S, et al. Effectiveness of subgingival irrigation of an indigenous 1% curcumin solution on clinical and microbiological parameters in chronic periodontitis patients: A pilot randomized clinical trial. Contemporary Clin Dent 2013; 4:186-191.
- Hung HC, Douglass CW. A meta-analysis of the effect of scaling and root planing, surgical treatment, and antibiotic therapies on periodontal probing depth and attachment loss. J Clin Periodontol 2002; 29:975–986.
- Gruenwald O, Brendle T, Jaenicke C. PDR for herbal medicine. Med Economics Company 1998; 875-879.
- Cardoso-Ugarte GA, López-Malo A, Sosa-Morales ME. Cinnamon (Cinnamomum zeylanicum) essential oils. In Essential oils in food preservation, flavor, and safety. Academic Press 2016; 339-347.
- Dong Y, Lu N, Cole RB. Analysis of the volatile organic compounds in Cinnamomum cassia bark by direct sample introduction thermal desorption gas chromatography-mass spectrometry. J Essential Oil Res 2013; 25:458-463.
- Mamajiwala AS, Sethi KS, Raut CP, et al. Comparative evaluation of chlorhexidine and cinnamon extract used in dental unit waterlines to reduce bacterial load in aerosols during ultrasonic scaling. Indian J Dent Res 2018; 29:749.
- Gupta D, Jain A. Effect of cinnamon extract and chlorhexidine gluconate (0.2%) on the clinical level of dental plaque and gingival health: A 4-week, triple-blind randomized controlled trial. J Int Acad Periodontol 2015; 17:91-98.
- Holt JG, Krieg NR, Sneath PH, et al. Bergey’s manual of determinative bacteriology. 9th Edn. Baltimor: William & Wilkins 1994.
- Mahmoudpour A, Rahimi S, Sina M, et al. Isolation and identification of Enterococcus faecalis from necrotic root canals using multiplex PCR. J Oral Sci 2007; 49:221-227.
- Barnard JP, Stinson MW. The alpha-hemolysin of Streptococcus gordonii is hydrogen peroxide. Infect Immun 1996; 64:3853–3857.
- Harley JP, Prescott LM. Laboratory exercises in microbiology. 5th Edn. McGraw-Hill Companies 2000.
- Garcia-Garrote F, Cercenado E, Bouza E. Evaluation of a new system, VITEK 2, for identification and antimicrobial susceptibility testing of Enterococci. J Clin Microbiol 2000; 38:2108–2111.
- Andrews JM. Determination of minimum inhibitory concentrations. J Antimicrob Chemotherapy 2001; 48:5-16.
- Bakkali F, Averbeck S, Averbeck D, et al. Biological effects of essential oils-a a review. Food Chem Toxicol 2008; 46:446-475.
- Maarit K, Tuulia H. Comprehensive two-dimensional liquid chromatography in analysis of Lamiaceae herbs: Characterisation and quantification of antioxidant phenolic acids. J Chromatography 2007; 1145:155–164.
- Tajkarimi MM, Ibrahim SA, Cliver DO. Antimicrobial herb and spice compounds in food. Food control 2010; 21:1199-1218.
- Farag R, Hewedi F. Antimicrobial activity of some Egyptian spice essential oils. J Food Prot 2003; 52: 665-667.
- Vallverdú-Queralt A, Regueiro J, Martínez-Huélamo M, et al. A comprehensive study on the phenolic profile of widely used culinary herbs and spices: Rosemary, thyme, oregano, cinnamon, cumin and bay. Food Chem 2014; 154:299-307.
- Yassin MA, Moslem MA, El-Samawaty AMA, et al. Effectiveness of allium sativum in controlling sorghum grain molding fungi. J Pure Appl Microbiol 2013; 101.
- Shan Bl, Cai YZ, Sun M, et al. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J Agric Food Chem 2007; 53:7749–7759.
- Filoche K, Soma K, Sissone C. Antibacterial effects of essentrial oils in combination with chlorhexidine digluconate. Oral Microb Immuno J 2005; 20:221-225.
- Å?imÅ?ek M, Duman R. Investigation of effect of 1, 8-cineole on antimicrobial activity of chlorhexidine gluconate. Pharmacognosy Res 2017; 9:234.
- Haffajee AD, Yaskell T, Socransky SS. Antimicrobial effectiveness of an herbal mouthrinse compared with an essential oil and a chlorhexidine mouthrinse. J Am Dent Assoc 2008; 139:606-611.
- Lang N, Brecx MC. Chlorhexidine digluconate–an agent for chemical plaque control and prevention of gingival inflammation. J Periodont Res 1986; 21:74-89.
- Moran JM. Home-use oral hygiene products: mouthrinses. Periodontol 2008; 48:42-53.
- Whatmore AM, Efstratiou A, Pickerill AP, et al. Genetic relationships between clinical isolates of streptococcus pneumoniae, streptococcus oralis, and streptococcus mitis: characterization of “atypical” pneumococci and organisms allied to S. Mitis HarboringS. Pneumoniae virulence factor-encoding genes. Infection Immunity 2000; 68:1374-1382.
- Kilian M. Streptococcus and Lactobacillus. In: Topely and Wilson’s microbiology and microbial infections. 2005.
- Kolenbrander PE. Oral microbial communities: Biofilms, interactions, and genetic systems. Annu Rev Microbiol 2000; 54: 413–437.
- Valm AM. The structure of dental plaque microbial communities in the transition from health to dental caries and periodontal disease. J Mol Biol 2019; 431:2957-2969.
- Heller D, Helmerhorst EJ, Gower AC, et l. Microbial diversity in the early in vivo-formed dental biofilm. Applied Environ Microbiol 2016; 82:1881-1888.
- Lakade LS, Shah P, Shirol D. Comparison of antimicrobial efficacy of chlorhexidine and combination mouth rinse in reducing the Mutans streptococcus count in plaque. J Indian Society Pedodont Preventive Dent 2014; 32:91.
- Graziani F, Gabriele M, D'Aiuto F, et al. Dental plaque, gingival inflammation, and tooth-discolouration with different commercial-formulations of 0.2% chlorhexidine rinse: A double-blind randomised controlled clinical trial. Oral Health Prev Dent 2015; 13:101.
- Ali-Shtayeh MS, Abu Ghdeib SI. Antimycotic activity of twenty-two plants used in folkloric medicine in the Palestinian area for the treatment of skin diseases suggestive of dermatophyte infection. Mycoses 1999; 42:665-672.
Author Info
Ahmed Abdulwahid Hasan and Ayser Najah*
Department of Periodontics, College of Dentistry, University of Baghdad, IraqCitation: Ahmed Abdulwahid Hasan, Ayser Najah, Antibacterial Activity of Alcoholic Cinnamon Extract and Chlorhexidine on Streptococcus Mitis as Primary Colonizers in Periodontal Disease, J Res Med Dent Sci, 2021, 9 (4):276-284.
Received: 20-Mar-2021 Accepted: 12-Apr-2021