Research - (2019) Volume 7, Issue 6
An In Vitro Assessment of Microleakage under Stainless Steel Bracket Bonded with Three Different Light-Cures Orthodontic Adhesives After Thermocycling and Water Storage
Omaima Lateef Salman* and Reem Atta Rafeeq
*Correspondence: Omaima Lateef Salman, Department of Orthodontics, College of Dentistry, University of Baghdad, Baghdad, Iraq, Email:
Abstract
Background: This in vitro study was conducted to assess the microleakage under stainless steel brackets bonded with three different adhesive systems at both adhesive interfaces occlusally and gingivally after thermocycling and two-month water storage duration.
Materials and methods: A total of 48 sound human upper premolar teeth were selected and arbitrarily allocated into 3 equal groups on the basis of the adhesive system used as; T, S and G groups of 16 teeth each, group T, Transbond XT adhesive; group S, Transbond XT self-etching primer; group G, resin-modified glass ionomer adhesive (GC Fuji ORTHO LC). All specimens in groups T, S and G were framed in different colored coded acrylic blocks; pink, yellow and green, respectively. After bonding and 500 thermocycles, half of specimens of each subgroup (n=8), tested after thermocycling and 24 hours’ water storage while the 2nd half were stored in an incubator at 37°C to be tested after 2 months. After each storage durations, the bonded samples were immersed in 2% methylene blue solution for 24 hours, sectioned longitudinally and then examined by stereomicroscope to measure the dye penetration at the enamel-adhesive and bracket-adhesive of the occlusal and gingival margins. Statistical analysis was performed using the Kruskal-Wallis test and the Mann-Whitney U-test and Wilcoxon signed rank test.
Results: After both storage duration, highly significant differences showed between adhesives groups at both adhesive interfaces and GC Fugi ORTHO LC displayed more microleakage than other adhesives at different interfaces. The gingival margin of all groups exhibited higher microleakage values compared with that of occlusal margin for both adhesive interfaces. The microleakage increased after 2 months water storage in all adhesive groups, but without significant differences.
Conclusion: The type of adhesive may play an important role in the microleakage event and GC Fugi ORTHO LC exhibited more microleakage than other adhesives at both adhesive interfaces and at bracket margins, Thermocycling and 2 months water storage duration not significantly increase the microleakage in different tested groups.
Keywords
Microleakage, Dye penetration, Adhesive, Thermocycling, Water storage
Introduction
In restorative dentistry, the microleakage is the dissemination of oral fluids and bacteria along the tooth-restoration interface [1]. From the orthodontic perspective, micro-leakage is a substantial problem in fixed appliance treatment, represents the reduction in the marginal integrity that facilitates the development of white-spot lesions around and under the bracket surface area and may lead to bond failure [2,3].
The polymerization shrinkage is one of the major drawbacks of light-cure composite and the main cause of microleakage [4]. Recently, studies on the development of bonding systems have increased to achieve the durable bond between an orthodontic attachment and enamel surface, among these new bonding systems, selfetching primers (SEPs) that simplify bracket bonding stages, save chair time and decrease saliva contamination [5]. Other new materials are the light cure resin-modified glass ionomer cements which consider as long-term fluoride releasing and recharging agents with less damaging effects on enamel surface [6]. Despite these advances, the demineralization due to microleakage and bond failure continue to affect the orthodontic treatment outcome. Numerous studies in orthodontics showed some degree of microleakage around and under bracket [4-7] and bands [8].
In addition to potopolymerization shrinkage, bonding materials are subjected to complex oral milieu effect on the durability of the bond by altering the physical and mechanical properties of adhesive and bracket materials, and the oral temperature changes induced by the food and beverages ingestion also lead to volumetric changes and adhesive joint fatigue, which consequently lead to microleakage [9]. Some studies have used thermocycling as an artificial aging method. However, the most commonly used artificial aging method, especially in restorative dentistry, is the water storage. The water storage effect on bond durability by hydrolytic degradation of the interface components [10]. Generally, most bonding studies favored the testing after 24 hours, as it has permitted comparison with other in vitro studies [11] and polymerization is anticipated to be completed at the end of 24 hours. However, this time period of 24 hours does not reproduce clinical orthodontic practice in which the archwire is typically placed after bonding [12]. On the other hand, significant degradation of the adhesive and its bond to tooth enamel would have occurred over time. Thus, this study was designed to investigate and compare the amount of microleakage beneath stainless steel brackets bonded with three different adhesive materials and estimate the effect of thermocycling and two months’ water storage on microleakage values.
Material and Methods
A total of 75 human upper first premolars extracted for orthodontic purposes were collected. After cleaning and examining the collected teeth with 10X magnifying lens, only 48 teeth were selected with no caries, cracks or restorations, and had not subjected to chemical pretreatment [13]. The specimens were mounted in different color-coded self-cured acrylic blocks measuring 1.5 × 1.5 × 2 cms. After mounting, the specimens were stored in distilled water to avert dehydration until bonding process [14].
The standard edge wise stainless steel brackets (Ortho Technology Company, USA) were used and the specimens were divided into three groups:
Group T: 16 specimens bonded with Transbond XT adhesive with pink color-coded.
Group S: 16 specimens bonded with Transbond XT self-etching primer with yellow color-coded.
Group G: 16 specimens bonded with GC Fuji ORTHO LC with green color-coded.
Bonding procedure
The buccal surface of all specimens was polished with fluoride-free pumice slurry with a rubber cup for 10 seconds [15]. Then, the bonding procedure recommended by the manufacturer was followed for each group.
Group T: 37% phosphoric acid etching gel (SDI, California, USA) for 30 seconds applied to the enamel surface then was rinsed with water for 30 seconds and dried to get a frosty white appearance of tooth surface. Transbond XT primer was coated on the etched tooth surface and thinned with a gentle air burst for 1-2 seconds.
Group S: A self-etching primer (Transbond Plus, 3M, unitek, Monrovia, USA) was applied on buccal surface for a minimum 3-5 sec., then thinned with a gentle air burst for 1-2 seconds. Transbond XT adhesive paste (3M Unitek) was used for bracket bonding in groups T and S.
Group G: GC Fuji ORTHO LC (GC Company, Tokyo, Japan) used for RMGIA system, 20% polyacrylic acid was applied for 20 seconds to the bonding surface of the tooth then rinsed thoroughly with water. The cement was mixed in ratio of 3.6g/1.0 g of the powder and liquid, one level scoop of powder to two drops of liquid.
For all adhesive groups, the bonding base of the brackets was loaded with adhesive and placed on enamel surface. A load of 200 g was applied to each bracket by a surveyor with simple modification to standardize the adhesive thickness [16]. Excess adhesive around the brackets was removed before polymerization. The LED light source with intensity of 1200 mW/cm2 was used for curing adhesive for 10 seconds on each side i.e. mesial and distal [17]. Once of the bonding procedure was finished, all specimens were kept in distilled water for 24 hours, then subjected to 500 thermocycles in distilled water baths between 5 and 55°C following the ISO 11405 recommendations with a dwell time of 30 s and a transfer between baths took 5 and 10 seconds [15]. Then, half of specimens in each group tested immediately following thermocycling and 24 hours’ water storage. The remaining half of each group stored was incubated in distilled water to be tested after 2 months.
Microleakage testing
After each storage durations, the teeth were dried and coated with two layers of nail polish leaving up to 1 mm around the edges of the bracket base exposed. Afterwards, the samples were immersed in 2 percent solution of methylene blue for 24 hours at room temperature [17]. After 24 hours, the specimens were thoroughly rinsed to discard the remaining dye and the nail varnished was removed by a sharp instrument, and the tooth crown was blocked with clear acrylic resin. Then, four parallel cuts made longitudinally in occlusalcervical direction for each specimens [4] by using a water-cooled microtome (MT-4 Diamond cut-off saw, USA) (Figure 1), thus providing three sections for each tooth. Each section was examined on both sides, so that each specimen underwent six examinations. The dye penetration for each interface on the gingival and occlusal margins was measured in millimeters directly using the stereomicroscope at ×40 magnification with Image J® analyzer software (Figure 2) [17].

Figure 1: Specimen sectioning with microtome.
Figure 2: Measurement of dye penetration with ImageJ®.
Statistical analysis
For each specimen, the microleakage measured either in occlusal and gingival margins, along the adhesive–enamel and adhesive-bracket interface and was obtained by calculating the mean values from all sections. Data were analyzed using SPSS software version 21 by Kruskal-Wallis, Mann Whitney U-tests and Wilcoxon signed rank tests. In the statistical evaluation, the following levels of significance are used (Table 1).
| Non-significant | P>0.05 |
| Significant | 0.05 P>0.01 |
| Highly significant | P ≤ 0.01 |
P=Probability value
Table 1: Levels of significance.
To test the intra-examiner reliability, 5 specimens were measured twice in 1 week’s interval by same examiner by using percentage of agreement. Intraexaminer calibrations showed 100% agreement.
Results
All groups exhibited a variation in the amounts of microleakage, Figure 3 presented the microleakage median values at enamel–adhesive interface and adhesive-bracket interfaces of different adhesive systems after both water storage durations and showed higher levels of microleakage showed for C group at both adhesive interfaces and after both storage durations followed by that of S group and least median value seen for T group. Table 2 showed groups’ differences of micro leakage in different groups, Kruskal-Wallis test revealed highly significant differences (P ≤ 0.01) among all groups after both storage durations. The Mann Whitney U-test was used to test the median differences between each two groups and showed that after 24 hours’ storage duration, there were highly significant differences between T and S, T and G groups at both adhesive interfaces and the differences between S and G was significant at adhesivebracket interface but not significant at adhesiveenamel interface. After 2-month storage duration, there were significant differences between T and S groups, a high significant difference between T and G groups at adhesive-bracket and high significant differences between T and S, T and G groups at adhesive-enamel with no significant differences between S and G at any interfaces (Table 2).
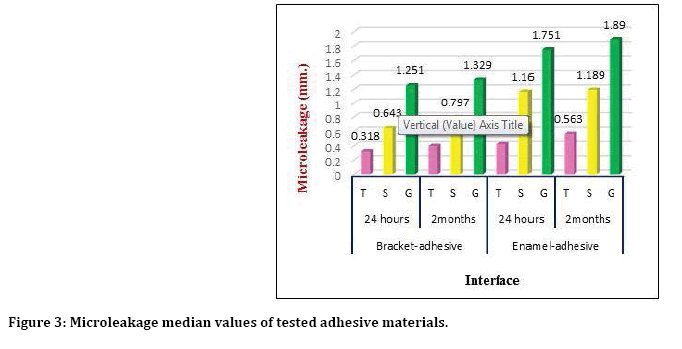
Figure 3: Microleakage median values of tested adhesive materials.
| Storageduration | Interface | Adhesives | Adhesive difference | ||||
|---|---|---|---|---|---|---|---|
| KWH test | MWU test | ||||||
| X2 test | p-value | Groups | MWU test | p-value | |||
| 24 hours | Bracket-Adhesive | T | 16.485 | 0.000** | T-S | 3 | 0.002** |
| S | T-G | 0 | 0.001** | ||||
| G | S-G | 11 | 0.027 * | ||||
| Enamel-Adhesive | T | 15.695 | 0.000** | T-S | 1 | 0.001** | |
| S | T-G | 1 | 0.001** | ||||
| G | S-G | 16 | 0.093 | ||||
| 2 months | Bracket-Adhesive | T | 12.875 | 0.002** | T-S | 8 | 0.012 * |
| S | T-G | 1 | 0.001** | ||||
| G | S-G | 18 | 0.141 | ||||
| Enamel-Adhesive | T | 14.285 | 0.001** | T-S | 7 | 0.009** | |
| S | T-G | 0 | 0.001** | ||||
| G | S-G | 15 | 0.074 | ||||
*Significant p<0.05; **Highly significant p ≤ 0.01
Table 2: Adhesive differences effect on microleakage median values (mm.) after both storage durations.
Table 3 showed the descriptive statistics and side differences of microleakage measurements at adhesive- bracket, adhesive-enamel and showed the microleakage at gingival side higher than that of occlusal side with the highest value seen for G group at adhesive-enamel-interfaces after both storage durations and the significant differences (P<0.05) observed at bracket-adhesive in G group after 24 hours and S group after 2 months storage durations, while at enamel-adhesive interfaces, the significant difference was seen in T group after 24 hours, S group after 2 months, and G groups after both storage durations as revealed by Wilcoxon signed rank test (WSR). Table 4 showed the differences of total Microleakage measurements between the two storage duration; the microleakage increased after thermocycling and 2 months’ water storage but without significant differences for all tested groups (P>0.05).
| Interface | Duration | Adhesives | Descriptive statistics | Side | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Occlusal | Gingival | Side Difference | ||||||||
| Median | Mean | SD | Median | Mean | SD | WSR test | p-value | |||
| Bracket-Adhesive | 24 hours | T | 0.21 | 0.245 | 0.264 | 0.44 | 0.345 | 0.242 | -0.734 | 0.463 |
| S | 0.546 | 0.698 | 0.361 | 0.724 | 0.81 | 0.325 | -1.26 | 0.208 | ||
| G | 1.121 | 1.045 | 0.437 | 1.381 | 1.387 | 0.392 | -2.521 | 0.012* | ||
| 2 months | T | 0.334 | 0.281 | 0.254 | 0.466 | 0.502 | 0.431 | -1.352 | 0.176 | |
| S | 0.73 | 0.755 | 0.473 | 0.86 | 1.346 | 0.811 | -2.38 | 0.017* | ||
| G | 1.241 | 1.282 | 0.472 | 1.571 | 1.532 | 0.394 | -1.26 | 0.208 | ||
| Enamel-Adhesive | 24 hours | T | 0.341 | 0.311 | 0.226 | 0.715 | 0.725 | 0.457 | -2.366 | 0.018* |
| S | 1.185 | 1.22 | 0.488 | 1.327 | 1.421 | 0.624 | -1.352 | 0.176 | ||
| G | 1.483 | 1.479 | 0.502 | 2.152 | 1.94 | 0.502 | -2.521 | 0.012* | ||
| 2 months | T | 0.51 | 0.462 | 0.208 | 0.514 | 0.543 | 0.472 | -0.338 | 0.735 | |
| S | 0.872 | 0.939 | 0.585 | 1.378 | 1.769 | 0.947 | -2.521 | 0.012* | ||
| G | 1.74 | 1.903 | 0.433 | 2.098 | 2.142 | 0.417 | -1.96 | 0.049* | ||
*Significant p<0.05
Table 3: The descriptive statistics and effect of side difference on the microleakage (mm) of tested groups.
| Adhesives | Descriptive statistics | Time | ||||||
|---|---|---|---|---|---|---|---|---|
| 24 hours | 2 months | Difference | ||||||
| Median | Mean | S.D. | Median | Mean | SD | MWU test | p-value | |
| T | 0.422 | 0.406 | 0.215 | 0.411 | 0.447 | 0.232 | 30.5 | 0.875 |
| S | 0.951 | 1.038 | 0.398 | 1.152 | 1.203 | 0.61 | 29 | 0.753 |
| G | 1.528 | 1.463 | 0.435 | 1.6 | 1.715 | 0.336 | 21 | 0.248 |
Table 4: The differences of total microleakage between the two storage durations among tested groups.
Discussion
The unaesthetic and potentially irreversible white spot lesion is known as a serious side effect of fixed appliances treatment [18] and can developed in a month period after the start of treatment [19]. Therefore, the area beneath the bracket is critical and requires investigation as the area around a bracket [4] and the assessment of microleakage can be reflected the sealing ability of adhesive materials. The dye penetration test in this study used to assess microleakage among specimens, as it is the most frequently used, simple, cost-effective, and non-toxic [4,20].
The microleakage under brackets was investigated at the occlusal and gingival margins in the enamel-adhesive and adhesive-bracket interfaces like other in vitro studies [4,7], and the adhesive-enamel and adhesive-bracket interfaces were separately evaluated similar to previous studies [4,21]; hence, the adhesiveenamel interfaces are critical to the occurrence of white spot lesions and adhesive-bracket may play a pivotal role in bracket failure as a result of bond degradation [4]. Several studies reported the effect of microleakage on the bond strength of brackets and James et al. [22] could not demonstrate any relationship between microleakage and bond strength. The results of this study showed that the microleakage was detected in all groups and all interfaces after both water storage durations and there were highly significant differences among three adhesive materials. The Transbond XT causes least microleakage value and RMGIC showed the highest values among adhesive materials, these findings were not similar to those reported by Yagci, et al. [18], Aliks, et al. [2] who proved that the bonding materials or techniques did not affect the microleakage amount under brackets. Though, several factors may affect microleakage amount such as the type and composition of adhesive, enamel conditioning, bracket materials and base design, and coefficient of thermal expansions [17,23]. In addition, the higher microleakage of RMGIC and the possibility of enamel demineralization might be counteracted by effectiveness of this adhesive in re-mineralizing the demineralized enamel as revealed by Ramoglu, et al. [24]. The brackets bonded with Transbond XT Plus SEP showed significantly more microleakage than the conventional Transbond XT after both storage durations. These results are consistent with finding of Uysal, et al.[20] who stated that brackets bonded with self-etching primer systems displayed significantly higher microleakage at the enamel-adhesive interface gingivally and gave a plausible explanation to this results concerning the extent and depth of etching pattern, as the self‑etching systems create more uniform, less deep etch pattern as compared by longer resin tags and superior resin penetration to enamel of total etched systems that may limit the potential microleakage [25].
The microleakage values obtained from gingival margins higher than that obtained from occlusal side, these differences were statistically significant in most interfaces of tested groups. These finding are in accordance with those of Ramoglu et al. [24] who reported higher microleakage amounts at gingival sides and attributed that to the surface angulation of the buccal area that results in thicker adhesives at gingival margin.
The temperature fluctuation in oral cavity influences the adhesion between the adhesive material and the tooth, the difference in expansion coefficients within “bracket‑adhesive‑enamel” complex causes different dimensional changes by repeated expansion and contraction results in water or oral fluids being sucked in and pushed out at the bracket edges, this might cause the tooth fractures, cracks and consequent microleakage [26]; so that, the thermal cycling process was broadly used in this type of studies [9,27]. In addition, water storage can render the bonding effectiveness by hydrolysis degradation of the interface components [9]. The water can also infiltrate and weaken the mechanical characteristics of the polymer matrix [10,28]. This have explained the increasing in microleakage amount for different three adhesive systems after thermocycling and 2 months’ water storage but it not statistically significant may be due to insufficient time for observing the changes. However, clinical conditions may differ significantly in vivo, it is impossible to extrapolate exactly the result of an in-vitro study to the actual oral environment.
Conclusion
Microleakage was detected in all the investigated adhesive groups and the type of adhesives may play an important role in the microleakage event. GC Fugi Ortho LC showed more microleakage than other adhesive materials at both adhesive interfaces and at both occlusal and gingival margins, Transbond XT showed the lowest microleakage values as compared with GC Fugi Ortho LC and TSEPs. The gingival margins demonstrated higher amount of microleakage than occlusal margins at both adhesive interfaces. Thermocycling and 2 months’ water storage duration not significantly increase the microleakage in different tested groups.
References
- Fabianelli A, Pollington S, Davidson CL, et al. The relevance of microleakage studies. Int Dent 2007; 9:64-74.
- Alkis H, Turkkahraman H, Adanir N. Microleakage under orthodontic brackets bonded with different adhesive systems. Eur J Dent 2015; 9:117–21.
- Moosavi H, Ahrari F, Mohamadipour H. The effect of different surface treatments of demineralized enamel on microleakage under metal orthodontic brackets. Prog Orthod 2013; 14:2-9.
- Arhun N, Arman A, Cehreli SB, et al. Microleakage beneath ceramic and metal brackets bonded with a conventional and an antibacterial adhesive system. Angle Orthod 2006; 76:1028-1034.
- Minick GT, Oesterle LJ, Newman SM, et al. Bracket bond strengths of new adhesive systems. Am J Orthod Dentofacial Orthop 2009; 135:771-776.
- Gange P. The evolution of bonding in orthodontics. Am J Orthod Dentofacial Orthop 2015; 147:56-63.
- Arikan S, Arhun N, Arman A, et al. Microleakage beneath ceramic and metal brackets photopolymerized with LED or conventional light curing units. Angle Orthod 2006; 76:1035-1040.
- Gillgrass T, Millett DT, Creanor SL, et al. Fluoride release, microbial inhibition and microleakage pattern of two orthodontic band cements. J Dent 1999; 27:455-461.
- Canbek K, Karbach M, Gottschalk F, et al. Evaluation of bovine and human teeth exposed to thermocycling for microleakage under bonded metal brackets. J Orofac Orthop 2013; 74:102-112.
- De Munck J, Van Landuyt K, Peumans M, et al. A critical review of the durability of adhesion of tooth tissue: methods and results. J Dent Res 2005; 84:118-132.
- Aljubouri YD, Millett DT, Gilmour WH. Laboratory evaluation of a self-etching primer for orthodontic bonding. Eur J Orthod 2003; 25: 411–415.
- Bishara SE, VonWald L, Laffoon JF, et al. Effect of a self-etch primer/adhesive on the shear bond strength of orthodontic brackets. Am J Orthod Dentofacial Orthop 2001; 119:621–624.
- Bishara SE, Ostby AW, Laffoon JF, et al. Shear bond strength comparison of two adhesive systems following thermocycling: A new self-etch primer and a resin-modified glass ionomer. Angle Orthod 2007; 77:337-341.
- Uysal T, Ulker M, Ramoglu SI, et al. Microleakage under metallic and ceramic brackets bonded with orthodontic self-etching primer systems. Angle Orthod 2008; 186:1089-1094.
- Sabzevari B, Ramazanzadeh BA, Moazzami SM, et al. Microleakage under orthodontic metal brackets bonded with three different bonding techniques with/without thermocycling. J Dent Mater Tech 2013; 2:21-28.
- Bishara SE, Ostby AW, Laffoon J, et al, Enamel cracks and ceramic bracket failure during debonding. In Vitro. Angle Orthod 2008; 78:1078-1094.
- Atash R, Fneiche A, Cetik S, et al. In vitro evaluation of microleakage under orthodontic brackets bonded with different adhesive systems. Eur J Dent 2017; 11:180.-185.
- Yagci T, Uysal T, Ulker M, et al. Microleakage under orthodontic brackets bonded with the custom base indirect bonding technique. Eur J Orthod 2010; 32:259–263.
- O’Reilly MM, Featherstone JD. Demineralization and remineralization around orthodontic appliances: An in vivo study. Am J Orthod Dentofacial Orthop 1987; 92:33‑40.
- Uysal T, Ulker M, Ramoglu SI, et al. Microleakage under metallic and ceramic brackets bonded with orthodontic self-etching primer systems. Angle Orthod 2008; 78:1089-1094.
- Vicente A, Ortiz AJ, Bravo LA. Microleakage beneath brackets bonded with flowable materials: Effect of thermocycling. Eur J Orthod 2009; 31:390-396.
- James JW, Miller BH, English JD, et al. Eff ects of high-speed curing devices on shear bond strength and microleakage of orthodontic brackets. Am J Orthod Dentofacial Orthop 2003; 123:555-561.
- Hamamci N, Akkurt A, Basaran G. In vitro evaluation of microleakage under orthodontic brackets using two different laser etching, self-etching and acid etching methods. Lasers Med Sci 2009; 27:1-6.
- Ramoglu SI, Uysal T, Ulker M, et al. Microleakage under ceramic and metallic brackets bonded with resin-modified glass Ionomer. Angle Orthod 2009; 79: 138-143.
- Cal-Neto JP, Miguel JA. Scanning electron microscopy evaluation of the bonding mechanism of a selfetching primer on enamel. Angle Orthod 2006; 76: 132-136.
- Sangamesh B, Amitabh K. Iatrogenic effects of orthodontic treatment: Review on white spot lesions. IJSER 2011; 2:1-16.
- Wahab F, Shaini F, Morgano S. The effect of thermocycling on microleakage of several commercially available composite class V restorations in vitro. J Prosthet Dent 2003; 90:168-174.
- Hashimoto M, Ohno H, Sano H, et al. Micromorphological changes in resin–dentin bonds after 1 year of water storage. J Biomed Mater Res 2002; 63:306–311.
Author Info
Omaima Lateef Salman* and Reem Atta Rafeeq
Department of Orthodontics, College of Dentistry, University of Baghdad, Baghdad, IraqCitation: Omaima Lateef Salman, Reem Atta Rafeeq, An In Vitro Assessment of Microleakage under Stainless Steel Bracket Bonded with Three Different Light-Cures Orthodontic Adhesives After Thermocycling and Water Storage, J Res Med Dent Sci, 2019, 7(6): 113-119.
Received: 27-Nov-2019 Accepted: 19-Dec-2019
