Research - (2020) Advances in Dental Surgery
Advanced Platelet Rich Fibrin APRF Comparison of Lymphocyte Cellularity as an Age Indicator Between Two Age Groups
Nauma Hafeez1, Radhika Arjunkumar1 and Abilasha R2*
*Correspondence: Abilasha R, Department of Oral Pathology, Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Sciences, Chennai, Tamil Nadu, India, Email:
Abstract
The study assesses the cells in platelet-rich fibrin using advanced platelet-rich fibrin and standard platelet-rich fibrin protocol between two age groups. Platelet-rich fibrin (PRF) is a fibrin matrix in which various cells are trapped including platelets and growth factors. It was first developed by Choukron et al. in France in the year 2001, It is a second-generation platelet concentrate developed for use specifically in oral and maxillofacial surgery. It is known to have various regenerative and healing properties including tissue regeneration, revascularization, osteogenic properties, etc. making it ideal for wound healing. PRF can be classified into two types based on the centrifugation protocol as standard–platelet-rich fibrin (S-PRF) and advanced platelet-rich fibrin (A-PRF). This study required volunteers from two different age groups: younger age group between 18–25 years and older age group which was more than 50 years. Histological analysis reveals the following: Monocyte quantity is similar in both S-PRF and A-PRF. Neutrophils are comparatively higher in the above 50 age group. Lymphocytes (S-PRF) more in older than younger age groups. Cells more distributed in A-PRF and concentrated in S-PRF. Loose fibrin in S-PRF and dense fibrin in A-PRF. Although it was found that the older age group could prove to have better properties in PRF, the results are inconclusive due to the limited sample size. Further research is required to enable optimal scaffold formation to be tailored for specific clinical applications.
Keywords
A-PRF, S-PRF, Choukroun PRF, Ghanaati PRF, Platelet concentrates
Introduction
Wound healing is an important part of medical and dental treatment. Periodontal treatment and various other dental procedures for that matter rely ultimately on tissues healing for its success. Procedures with tissue damage range from scaling and root planning all the way to complex procedures such as flap surgery, and tooth extraction [1], which involves justified tissue damage in order to remove the disease stimulant. Healing following such damage is crucial to the overall prognosis of the treatment. Over the years surgical wounds have been treated with various medicaments and dressings to aid in its healing [2]. The main objective in wound healing is to re-establish the epithelial covering [3], this is done readily by the body the moment there is a break in its continuity, although in cases of extensive wounds the body will require additional outside help.
The earliest way of dealing with extensive nonhealing wounds was by amputation [4,5] which is a very radical treatment option and therefore not preferred or readily accepted by patients, with the advent of grafts and wound disinfection methods, the former was used only as a last resort. Grafts for large wounds however were difficult to procure, cross-reaction and graft rejection was also a major concern when it came to using non-autografts. The need for better techniques in wound healing led to the advent of research in the field of regenerative healing.
In the year 2001, Curie et al. reviewed the role of fibrin glue in tissue-engineered skin grafts, over the years its effectiveness had been proven, a few years later fibrin sheets came into the market and produced promising results [6]. The role of fibrin in wound healing has been extensively studied and researched over the years [7], It is now used widely in regenerative healing. Another important component of wound healing is the platelets the use of platelets to treat wounds has been used since 1985 [8], its use is attributed to its various components including growth factors. Over the years various efforts have been made to combine fibrin and platelets for ultimate wound healing. The first generation platelet concentrate, namely the PRP (plateletrich plasma) combines the properties of fibrin and platelets [9], it proved successful in healing wounds, however, the preparation of PRP involves the addition of bovine thrombin and hence may induce allergic reactions or denial from patients having religious concerns. To overcome these drawbacks a second-generation platelet concentrate was developed in 2001, the platelet-rich fibrin. With further research and need to find a superior platelet product, a recent third-generation platelet concentrate was developed which is platelet-rich in growth factors (PRGF) [10], having added growth factor associated effects, however, the preparation does involve the addition of other components, and therefore PRF is preferred over PRGF in terms of patient compliance and ease of preparation. In this study, we will be focusing solely on PRF and comparing its properties between ages.
Platelet-rich fibrin (PRF) is a fibrin matrix in which various cells are trapped including platelets and growth factors. It was first developed by Choukron et al. [11] in France. It is a secondgeneration platelet concentrate developed for use specifically in oral and maxillofacial surgery limiting its use to the dental field [12]. It is known to have various regenerative and healing properties including tissue regeneration, revascularisation, osteogenic properties, etc. making it ideal for wound healing [13]. Since PRF is sourced directly from the patient itself with no need for additional chemical manipulation, it is a purely autologous material making it highly biocompatible [14]. PRF can be classified into two types based on the centrifugation protocol as standard–platelet-rich fibrin (S-PRF) and advanced platelet-rich fibrin (A-PRF). S-PRF is made by centrifugation of blood at a speed of 2700 rpm for 12 minutes and A-PRF is made at a centrifugation speed of 1500 for 14 minutes, the differences in the speed and time of centrifugation cause varying cellular distribution and diffusion pattern and hence varying regenerative results [12].
Platelet concentrates can be used for sinus lifts, socket preservation, intra-bony defects, jaw reconstruction, gingival grafts, etc [9]. Its vast application drives the need for further research in terms of age, gender, race, etc in search of a superior product. Understanding its cellular components can help create PRF specific for patients’ needs.
Previously we have worked on plenty of topics in periodontology [15-27]. Now we are planning to analyze the differences in A-PRF and S-PRF between the younger and older age groups to bring light upon which age group has superior tissue regeneration properties.
Materials and Methods
This study required volunteers from two different age groups: younger age group between 18–25 years and older age group which was more than 50 years. The study was approved by the institutional ethical committee. Each volunteer was informed of the study and written informed consent was taken. 20 ml of blood was taken from each volunteer, 10 ml of which was centrifuged according to the Choukron protocol to give S-PRF (2700 rpm for 12 minutes), and the other 10 ml according to the Ghanaati protocol to give A-PRF (1500 rpm for 14 minutes). The volunteers were selected based on the inclusion and exclusion criteria.
The inclusion criteria: Systemically healthy, Periodontally healthy Patients.
The exclusion criteria: Not within the selected age groups, Subjects with systemic diseases, Periodontal diseases.
A centrifuge machine was used with setting to adjust time and speed. Glass tubes were used for the collection of blood and centrifugation (helps facilitate clot formation). The PRF specimen was collected and stored in a 10% formalin solution for 24 hrs followed by sectioning and fixation. Hematoxylin and eosin stains were used to stain the slides and visualized under light microscopy. Histological cell-based evaluation was done. The date was tabulated and analyzed.
Results and Discussion
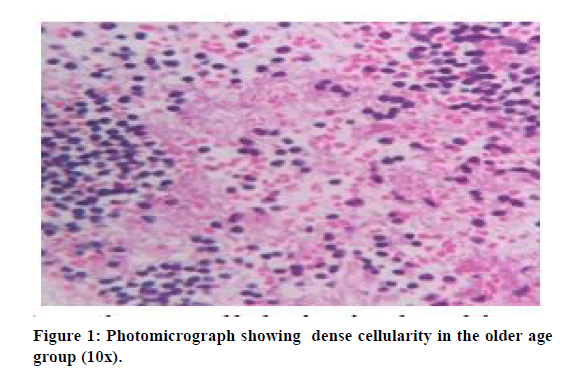
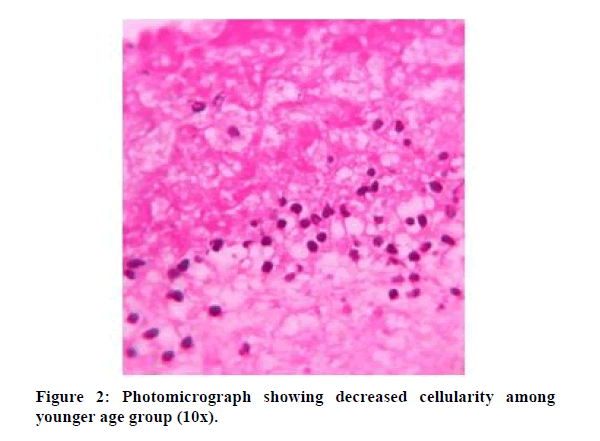
Cellular histological analysis was done based on three cell types which were lymphocytes, monocytes, and neutrophils. The following findings were noted. Monocyte quantity is similar in both S-PRF and A-PRF. Neutrophils are comparatively higher in the above 50 age group. Lymphocytes (S-PRF) more in older than younger age groups. Cells more distributed in A-PRF and concentrated in S-PRF. Loose fibrin in S-PRF and dense fibrin in A-PRF the following Tables 1 and 2 shows the cellular distribution between old and young age groups. Figures 1 and 2 show dense cellularity in the older age groups and reduced cellularity in the younger age groups, respectively.
Figure 1: Photomicrograph showing dense cellularity in the older age group (10x).
Figure 2: Photomicrograph showing decreased cellularity among younger age group (10x).
| Neutrophils | Lymphocytes | Monocytes | |||
|---|---|---|---|---|---|
| A-PRF | S-PRF | A-PRF | S-PRF | A-PRF | S-PRF |
| Moderate | Moderate | Moderate | Severe | Mild | Mild |
| Moderate | Moderate | Moderate | Severe | Mild | Mild |
| Mild | Moderate | Moderate | Moderate | Mild | Mild |
Table 1: Histological data for the age group above 50 years.
| Neutrophils | Lymphocytes | Monocytes | ||||
|---|---|---|---|---|---|---|
| A-PRF | S-PRF | A-PRF | S-PRF | A-PRF | S-PRF | |
| 1 | Mild | Mild | Moderate | Mild | Mild | Mild |
| 2 | Mild | Mild | Moderate | Mild | Mild | Mild |
| 3 | Mild | Moderate | Mild | Moderate | Mild | Mild |
Table 2: Histological data for the age group between 18–25 years.
It is a well-known fact that neutrophils help in the conversion of monocytes to macrophages which ultimately help in the regenerative properties. Hence higher the neutrophils better the regenerative properties. In this study it is found that the older age group have higher neutrophil count than the younger age group in both A-PRF and S-PRF, hence though not conclusive due to limited sample size it removed the preconceived notion of younger the better and hence motivates the use of PRF from older age groups and patients in general. One study comparing the PRF membrane between ages showed that the membrane is larger in older patients [14] when compared to the younger age, reinforcing the previous statement. The other differences such as loose or dense fibrin and concentrated or diffuse cells can be used according to the presentation of the problem to be solved for example a denser fibrin tissue to cover extensive wounds which are used aggressively like the vestibules or gingiva as compared to less used and relatively stationary tissues of the palate. Hence PRF can be specifically tailored to meet the various needs.
Previous studies were done on PRF and its age comparison shows similar results [28]. However, the fibrin density was found to be dissimilar. While this study shows loose fibrin network in S-PRF and dense fibrin structure in A-PRF, the study conducted by Ghanaati et al. [29] shows otherwise. However, the limited sample size of this study does pose a limitation to the above statement.
Conclusion
This study was taken up as an exploratory study. The results are inconclusive due to limited sample size and therefore further research with increased sample size and histochemical analysis is required, regardless it was found that older age groups could prove to have better properties in PRF when compared to a younger age. This encourages the use of PRF from older age groups removing the skeptics that surround it. This concept enables the optimal scaffold or composites to be tailored for specific clinical applications.
Acknowledgement
This research was supported by Saveetha institute of medical and technical sciences and has greatly assisted the research.
Conflict of Interest
Nil.
References
- Danda AK, Tatiparthi MK, Narayanan V, et al. Influence of primary and secondary closure of surgical wound after impacted mandibular third molar removal on postoperative pain and swelling—A comparative and split mouth study. J Oral Maxillofac Surg 2010; 68:309–312.
- Deodhar AK, Rana RE. Surgical physiology of wound healing: a review’, Journal of postgraduate medicine, 1997; 43:52–56.
- Theoret CL. Update on wound repair. Clin Techniques Equine Practice 2004; 3:110–122.
- Wangensteen OH, Smith J, Wangensteen SD. Some highlights in the history of amputation reflecting lessons in wound healing. Bulletin History Med 1967; 41:97–131.
- Manring MM, Hawk A, Calhoun JH, et al. Treatment of war wounds: A historical review. Clin Orthop Related Res 2009; 467:2168–2191.
- Sharpe JR, Jordan NR, Currie LJ. Enhancing skin epidermal stability. Biomaterials Treating Skin Loss 2009; 2:124–141.
- Clark RA. Fibrin and wound healing. Annals New York Academy Sci 2001; 936:355–367.
- Nurden AT, Nurden P, Sanchez M, et al. Platelets and wound healing. Frontiers Biosci 2008; 13:3532–3548.
- Ehrenfest DM, Andia I, Zumstein MA, et al. Classification of platelet concentrates (Platelet-Rich Plasma-PRP, Platelet-Rich Fibrin-PRF) for topical and infiltrative use in orthopedic and sports medicine: Current consensus, clinical implications and perspectives. Muscle Ligaments Tendons J 2019; 4:03.
- Pal US, Mohammad S, Singh RK, et al. Platelet-rich growth factor in oral and maxillofacial surgery. National J Maxillofac Surg 2012; 3:118–123.
- Dohan DM, Choukroun J, Diss A, et al. (2006) Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endodont 2006; 101:37–44.
- Preeja C, Arun S. Platelet-rich fibrin: Its role in periodontal regeneration. Saudi J Dent Res 2014; 5:117–122.
- Rao SG, Bhat P, Nagesh KS, et al. Bone regeneration in extraction sockets with autologous platelet rich fibrin gel. J Maxillofac Oral Surg 2013; 12:11–16.
- Borie E, Oliví DG, Orsi IA, et al. Platelet-rich fibrin application in dentistry: A literature review. Int J Clini Exp Med 2015; 8:7922–7929.
- Ramesh A, Ravi S, Kaarthikeyan G. Comprehensive rehabilitation using dental implants in generalized aggressive periodontitis. J Indian Society Periodontol 2017; 21:160–163.
- Ravi S, Malaiappan S, Varghese S, et al. Additive effect of plasma rich in growth factors with guided tissue regeneration in treatment of intrabony defects in patients with chronic periodontitis: A split-mouth randomized controlled clinical trial. J Periodontol 2017; 88:839–845.
- Arjunkumar R. Nanomaterials for the management of periodontal diseases in Chaughule, RS. (Edn.) Dental Applications of Nanotechnology. Cham: Springer International Publishing 2018; 203–215.
- Jain M, Nazar N. Comparative evaluation of the efficacy of intraligamentary and supraperiosteal injections in the extraction of maxillary teeth: A randomized controlled clinical trial. J Contemporary Dent Practice 2018; 19:1117–1121.
- Kavarthapu A, Thamaraiselvan M. Assessing the variation in course and position of inferior alveolar nerve among south Indian population: A cone beam computed tomographic study. Indian J Dent Res 2018; 29:405–409.
- Ramamurthy J. Comparison of effect of hiora mouthwash versus chlorhexidine mouthwash in gingivitis patients: A clinical trial. Asian J Pharm Clin Res 2018; 11:84–88.
- Ramesh A, Varghese S, Jayakumar ND, et al. Comparative estimation of sulfiredoxin levels between chronic periodontitis and healthy patients - A case-control study. J Periodontol 2018; 89:1241–1248.
- Ramesh A, Vellayappan R, Ravi S, et al. Esthetic lip repositioning: A cosmetic approach for correction of gummy smile-A case series. J Indian Society Periodontol 2019; 3:290–294.
- Ezhilarasan D, Apoorva VS, Ashok Vardhan N. Syzygium cumini extract induced reactive oxygen species-mediated apoptosis in human oral squamous carcinoma cells. J Oral Pathol Med 2019; 48:115–121.
- Kaarthikeyan G, Jayakumar ND, Sivakumar D. Comparative evaluation of bone formation between prf and blood clot alone as the sole sinus-filling material in maxillary sinus augmentation with the implant as a tent pole: A randomized split-mouth study. J Long-Term Effects Med Implants 2019; 29:105–111.
- Kavarthapu A, Malaiappan S. Comparative evaluation of demineralized bone matrix and type II collagen membrane versus eggshell powder as a graft material and membrane in rat model. Indian J Dent Res 2019; 30:877–880.
- Murthykumar K, Arjunkumar R, Jayaseelan VP. Association of vitamin D receptor gene polymorphism (rs10735810) and chronic periodontitis. J Investigative Clin Dent 2019; 10:e12440.
- Vijayashree Priyadharsini J. In silico validation of the non-antibiotic drugs acetaminophen and ibuprofen as antibacterial agents against red complex pathogens. J Periodontol 2019; 90:1441–1448.
- Saluja H, Dehane V, Mahindra U. Platelet-rich fibrin: A second generation platelet concentrate and a new friend of oral and maxillofacial surgeons. Annals Maxillofac Surg 2011; 1:53–57.
- Ghanaati S, Booms P, Orlowska A, et al. Advanced platelet-rich fibrin: A new concept for cell-based tissue engineering by means of inflammatory cells. J Oral Implantol 2014; 40:679–689.
Author Info
Nauma Hafeez1, Radhika Arjunkumar1 and Abilasha R2*
1Department of Periodontics, Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Sciences, Chennai, Tamil Nadu, India2Department of Oral Pathology, Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Sciences, Chennai, Tamil Nadu, India
Citation: Nauma Hafeez, Radhika Arjunkumar, Abilasha R, Advanced Platelet Rich Fibrin APRF Comparison of Lymphocyte Cellularity as an Age Indicator Between Two Age Groups, J Res Med Dent Sci, 2020, 8 (7): 339-343.
Received: 16-Oct-2020 Accepted: 05-Nov-2020 Published: 12-Nov-2020