Research - (2023) Volume 11, Issue 2
A study of anti-fungal resistance strains of Candida albicans yeast which isolated from clinical specimens of women Suffering from vaginal infections in Baqubah city
Yasser Muwafaq Mehdi*, Rabab Majead Abed and Hadi R. Rasheed Al-Taai
*Correspondence: Yasser Muwafaq Mehdi, Department of Biology, college of education for pure sciences, Iraq, Email:
Abstract
Vaginal swabs were collected from 40 ladies suffering from vaginal infections, their ages ranged between 20-50 years, attending the consulting clinic at Al-Batool Teaching Hospital in Baqubah for the period from December /2020 to February /2021.for married women. The samples that gave a positive test result for Candida albican infection were 20 samples The remaining 20 were negative. Of the 20 positive cases, 17 were pregnant women, their ages ranged between 20-30 years, and the non-pregnant women were 3 cases infected with Candida albicans, and their ages ranged between 31-50 years. Therefore, the infection rate of Candida albicans for the sample group was 80%, of which 59.4% were infected with C. albicans. Positive samples taken from pregnant women and 40.6% of samples taken from non-pregnant women. By using the Kirby-Bauer standard disk diffusion method, it was possible to assess the sensitivity and resistance of five C. albicans isolates to various antifungals. C. albicans had the highest level of resistance to various antifungals. Its resistance to Metroconazol (MZ), Fluconazol (FLU), and Ketoconazol (KCT) was all above 90%, but Amphotericin B (AMB) and Caspfungin (CAS) resistance rates were significantly lower at 25% and 45%, respectively. Antibiotic susceptibility testing of C. albicans for this study revealed that 12/20 (or 60%) of the isolates were multidrug resistant (MDR).
Keywords
Azols, albicans, resistance, sensitive
Introduction
Bloodstream infections are often caused either by key pathogenic organism Candida (BSIs). In research conducted in the US and Europe over the past 20 years, candidaemia has been listed as the fourth and seventh most frequent healthcare-associated BSI, respectively. One of the most frequent fungi that infect people are candida infections, which can affect the body's mucosa, skin, nails, and internal organs. In patients with impaired immune systems, Candida infections are a prevalent opportunistic illness. Mayer, et al. The incidence of candidiasis has been rising over the past ten years as a result of contemporary medical procedures, with fatality rates ranging from 40 to 50% [1].
In U.S. hospitals, Candida albicans is now the third most prevalent nosocomial pathogen. Adults commonly get vulvovaginal candidiasis; 70–75 percent of women encounter this infection at least once in their lifetime. VVC patients may experience vaginal discharge, which is typically described as white, creamy, curd-like (like cottage cheese), and adherent to the epithelium. However, the discharge could also be thin and loose, comparable to that seen in other vaginitis types [2].
Since candidiasis normally has a vaginal pH of 4 to 4.5, it can be distinguished from trichomoniasis or bacterial vaginitis, which both have elevated pH levels . Candida albicans is responsible for about 90% of vulvovaginal candidiasis and Candida glabrata for 5% of cases. Vulvovaginal candidiasis often affects pregnant women or women who use contraceptives because they contain estrogen, which raises glycogen levels, lactic acid bacteria, which breaks down glycogen, and mannoproteins, which enhances the likelihood of yeast adherence to mucous membrane [3-5]. Red ulcers with distinct edges, thick white or yellow discharges, burning, and discomfort in the infection location are all symptoms of infection. Thrush can be categorized as either uncomplicated or complicated, depending on whether the symptoms are mild to moderate and whether Candida albicans is the cause. Thrush may be referred to as complicated if it coexists with pregnancy, poorly managed diabetes, an immunological deficiency, four or more episodes of thrush in a year, or when any of these conditions are present. Additionally, complicated thrush may not be caused by the Candida albicans. Generally, allyl amines, azoles, echinocandins, morpholines, and polyenes are used as topical or systemic antifungal medications to treat C. albicans infections . Genes that code for enzymes playing significant roles in the pathway for ergosterol biosynthesis are disrupted by allylamines, azoles, and morpholines [5-7]. Fungi static azoles are among the various antifungals that are available, and because of their great efficacy, low cost, and minimal toxicity, they are frequently used to treat Candida infections. The widespread and sustained use of azoles, particularly fluconazole (FLU), is linked to the formation of azoleresistant Candida albicans strains.
The study's objective was to determine which antifungals from Candida albicans yeasts were the most responsive and resistant in women with vaginal infections.
Material and Methods
Collection of specimens
For the purpose of isolating candida, 40 samples of high vaginal swab (H.V.S.) were obtained using cotton swap media and loop full in the clinical labs of Al-BatouAl Teaching Hospital and Baquba Teaching Hospital between December 17, 2020, and February 17, 2021.
Direct examination
The standard procedure for examining all vaginal swab specimens was to mount them on a clean slide with a drop of KOH 10%, cover them with cover-slip, and then gently worm the slide (but not boiling). looked at under a microscope to look for growing Candida cells. To determine the isolates' response to stain, morphologies, arrangements, and yeast budding form, Gram stain was used to stain the samples [8-10].
Identification and Isolation of Candida albicans
Isolates
The specimens were struck directly on (SDAM) Sabouraud Dextrose Agar Medium and incubated at 37C for 48 hours (Al-Oebady, et al.). Following this, the positive results (Candida) were identified based on the morphological features on culture media and germ tub formation, and we then removed attachment from the resulting growth and struck it on (CAC) Chrom Agar Candida and incubated at 37Co for 48 hours [11-15]. Yeast isolation was then carried out.
Gem tub formation
By combining a little amount of an isolated colony with 0.5 ml of human serum, the ability of the yeast isolates to produce germ tubes was examined. For 2.5 hours, this suspension was incubated at 37°C.
As other yeast species may start to develop germ tubes, the incubation duration must not be longer than 3 hours. A cover slip was placed over a drop of the cultured serum and a microscope was used to check for the existence of germ tubes. In this test, serum was made by carefully aspirating blood from healthy human volunteers into vacutainer tubes without anticoagulants, incubating the samples in an upright position at room temperature for 30 minutes, followed by centrifuging the samples at 3000 rpm for 15 minutes [16-20]. The serum was then carefully transferred into sterilized tubes and defrosted at -8°C.
Susceptibility to Antibiotics Candida albicans
Setting up yeast suspension
Colony counts from a pure culture should be transferred using a sterile swab and suspended in 3 ml of sterile saline in a transparent test tube. The turbidity was compared to the McFarland tube, which contains to 1.5×108 CFU per milliliter.
Common Disc Diffusion Method
According to Magiorakos, et al. The susceptibility test of 7 antibiotics was conducted using the Kirby-Bauer method as a subordinate. The yeast suspension was made by selecting 4-5 isolated Candida albicans colonies from the native culture and putting them in a test tube with 4 ml of normal saline. This produced a fungal suspension with a mild turbidity that was comparable to the standard turbidity solution. A portion of the fungal suspension was carefully transferred and evenly dispersed over the Mueller-Hinton agar medium using a sterile cotton swab, then it was allowed to sit for 10 minutes [21-23].
The antifungal discs were then positioned on the agar and firmly pushed using a sterile forceps to ensure contact with the agar. The plates were then turned over and incubated for 18 to 24 hours at 37 degrees Celsius. With a ruler and the standard dimensions established by CLSI 2019 (Wayne, et al.) for Candida albicans, the zones of inhibition were measured [24].
Result Reading
Using a metric ruler, the diameters of the entire zone of inhibition were measured in millimeters after incubation. By comparing the diameter of each antibiotic disc's inhibition zone to the standard inhibition zone, sensitive, intermediate, and resistant groups were determined.
Results and Discussion
Gather samples
A total of 40 clinical specimens were gathered from vaginal swabs of a married female of various ages; of these, 40 (100%) tested positive for diverse species of Candida, whereas 20 isolates (50%) tested positive for Candida albicans. In this study, 40 patients with candidiasis between the ages of 21 and 5 years were assessed. The age range was divided into three groups: 21–30, 31–40, and 41–50 years. in the 21–30 age range 5/8 (62.5%) of the 50 patients with fungal infections in that age group had positive results for Candida albicans, while 5/10 (50%) of the patients with candidiasis in the age group of 31 to 40 had such results, and 10/22 (50%) of the patients with candidiasis in the age group of 41 to 50 had such results. Table 1 shows that the results obtained don't agree with more recent findings. They discover that patients aged 27 to 32 have the highest infection prevalence, followed by those aged 33 to 38 and those aged 21 to 26. Only 2 of 42 individuals (4.8%) between the ages of 39 and 45 have a yeast infection [25].
| Antifungal | Disc Concentration μg/disc | Diameter of inhibition zone (mm) | ||
|---|---|---|---|---|
| Resistant | Intermediate | Sensitive | ||
| Amphotericin B | 100 | <9 | 7-Oct | >18 |
| Ketoconazole | 30 | <30 | 31-35 | >37 |
| Metroconazol | 100 | <15 | 16-25 | >32 |
| Fluconazole | 10 | <27 | 28-38 | >40 |
| Caspofangin | 50 | <8 | 19-Sep | >25 |
Table 1: Lists the 2019 CLSI quality control standards for antifungal drugs
Recognizing Candida albicans from a vaginal swab
Candida albicans was identified from a High vaginal swab based on morphological characteristics of the culture media and germ tube production. Although several species were gathered for the current investigation, the most prevalent species will truly be the focus: Candida albicans, which appears as oval to spherical budding cells around epithelial cells, was identified using the KOH Direct quantity method by directly examining high vaginal swab specimens using 10% KOH. Numerous budding yeasts are seen on saline and KOH microscopy, however hypha components are not present [26]. The second phase in the identification process for Candida albicans was cultural traits. On Sabouraud dextrose agar, Candida colonies had the following characteristics: white to cream color, round, curved, soft and smooth to wrinkled shape, characteristic yeast odor; they proliferated quickly in 24 hours; and they matured in 3 days. Bhavan, et al. Concur with these findings.
The third step in the identification process was to examine the microscopic characteristics of Candida albicans in gram stain. According to Candida. albicans isolates were gram positive, round to oval, and had budding present Alshaikh, et al. (Figure 1).

Figure 1. yeast that B-budding, Gram stain of Candida albicans (40X) A-germ tube development.
Candida albicans identification using a Hichrom Agar medium
Candida colonies that developed on SDA were sub cultured on Hichrom agar media in order to identify Candida albicans. After an overnight incubation, the isolates grew well and produced colonies with different colors. According to the manufacturer's instructions, the colors of the colonies were observed and an assumptive identification was made of C. albicans, which was green, C. tropicalis, blue, C. krusei, pink colonies with a matte surface, C. norvegenensis, cream to pale pink, C. glabrata, pale cream, and C. famata, a light green Figure 2. With a sensitivity and specificity of 98%, the chromogenic medium Hichrom agar Candida provides a quick, easy way to identify common Candida species. The current investigation discovered that C. albicans could only be identified using Hichrom agar, the form and color of the Candida colonies on SDA and Hichromo candida agar are shown in Figure 2.
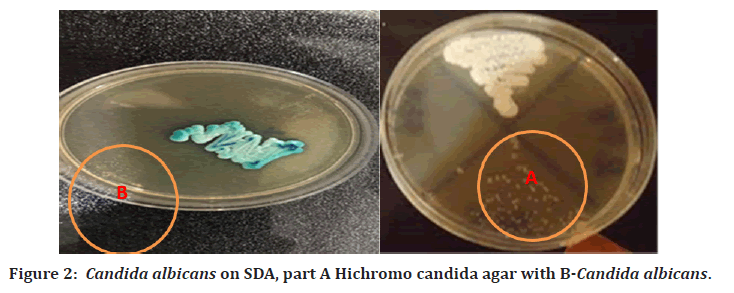
Figure 2. Candida albicans on SDA, part A Hichromo candida agar with B-Candida albicans.
Test for antifungal susceptibility
Based on the diameter of the inhibition zone (mm), which is listed in the materials and method table, the antifungal susceptibility of Candida albicans isolates was assessed using the disk diffusion method (Kirby-Bauer) according to the requirements of the Laboratory and Clinical Standards Institute CLSI (2019). Table 2 All 20 Candida albicans isolates underwent this test against five antifungal drugs from three classes: Fluconazol (FLU), Ketoconazol (KCT), Metroconazol (MZ), Amphotrecin B (AMB), and Caspfungin are examples of the azole class (CAS) [27]. As shown in Table 3 and Figures 3 for isolate C. albicans, the results demonstrated that all Candida albicans clinical isolates have a high level of resistance to the antifungal drugs tested. The table of the appendix also showed the various isolates' susceptibilities to these antibiotics.
| Age group in years | No. total positive specimens | No. positive specimens of Candida albicans | Percentage % |
|---|---|---|---|
| 21-30 | 8 | 5 | 62.50% |
| 31-40 | 10 | 5 | 50% |
| 41-50 | 22 | 10 | 50% |
| Total | 40 | 20 |
Table 2: Shows the specimen distribution by age group in years
| Anti-fungal | Number of Candida albicans (% ) total No.=20 | ||
|---|---|---|---|
| Resistant (R) | Moderate(M) | Sensitive(S) | |
| Amphotricin B | 5(25%) | 3(15%) | 12(60%) |
| Caspofangin | 9(45%) | 4(20%) | 7(35%) |
| Ketoconazol | 14(70%) | 0(0%) | 6(30%) |
| Fluconazol | 17(85%) | 0(0%) | 3(15%) |
| Metroconazol | 18(90%) | 0(0%) | 2(10%) |
Table 3: Antifungal Susceptibility

Figure 3. Patterns of Candida albicans antifungal susceptibility.
Antifungal susceptibility test results are shown in Table 3 by the percentage of resistant, moderate cases.
These findings are in agreement with Tamai, et al. Who found that while all Candida isolates showed high resistance to ketoconazole, fluconazole, and metroconazol, they were all susceptible to amphotericin B and caspofungin. The isolates (Candida albicans) were more responsive to amphotericin B, according to Ashour, et al. Also mentioned that all Candida isolates were amphotericin B susceptible. According to Kaur, et al. Out of the 103 Candida isolates, 6.7% of the isolates exhibited amphotericin B resistance, while 60 of the isolates (58.25%) exhibited fluconazole resistance (in contrast to 2.3% and 22.7% for Amphotericin B and Fluconazole, respectively, in the present investigation). Typically, Amphotericin B and caspofungin are effective against the majority of C. albicans strains. According to Lohse, et al. This medication has good in vitro efficacy against C. albicans biofilms. However, many Candida species, particularly C. albicans, now have higher rates of resistance to these antifungal medications, and the implications of this can be seen in the clinical situation. It is crucial to have antifungal medications that can treat these infections without causing greater resistance, which typically results from mutations in the pathway used for sterol biosynthesis, just like with azole treatments like fluconazole and clotrimazole. Tasca, et al. Although this has happened when azoles antifungal medications have been used against Candida species. CLSI breakpoints must change along with changes in the resistance of fungi to antifungal medications [28]. The largest category of antifungal medications, azoles, have been used for many years to treat infections brought on by Candida species. Candida species have developed more azole resistance recently, both in vitro and in clinical settings.
References
- Agarwal A, Xie B, Vovsha I, et al. Sentiment analysis of twitter data. In Proceedings of the workshop on language in social media 2011; 30-38.
- Al-Oebady MA. Isolation and identification of Candida species from vaginal, urine and oral swabs by chromagar Candida. Int j adv res 2015; 3:948-56.
- Alshaikh NA, Perveen K. Susceptibility of Fluconazole-Resistant Candida albicans to Thyme Essential Oil. Microorganisms 2021; 9:2454.
- Ashour SM, Kheiralla ZM, Maklad SS, et al. Relationship between virulence factors of Candida species with candiduria and myeloperoxidase concentrations. Int J Curr Microbiol App Sci 2015; 4:108-23.
- Bhavan PS, Rajkumar R, Radhakrishnan S, et al. Culture and Identification of Candida Albicans from Vaginal Ulcer and Separation of Enolase on SDS-PAGE. Int J Biol 2010; 2:84.
- Campoy S, Adrio JL. Antifungals. Biochem Pharmacol 2017; 133:86-96.
- Czechowicz P, Nowicka J, Gościniak G. Virulence factors of Candida spp. and host immune response important in the pathogenesis of vulvovaginal candidiasis. Int J Mol Sci 2022; 23:5895.
- Happel AU, Gasper M, Balle C, et al. Persistent, asymptomatic colonization with candida is associated with elevated frequencies of highly activated cervical Th17-like cells and related cytokines in the reproductive tract of South African adolescents. Microbiol Spectr 2022; 10:01626-01621.
- Kaur R, Goyal R, Dhakad MS, et al. Epidemiology and virulence determinants including biofilm profile of Candida infections in an ICU in a tertiary hospital in India. J Mycol 2014.
- Kumar A, Thakur VC, Thakur S, et al. Phenotypic characterization and in vitro examination of potential virulence factors of Candida species isolated from blood stream infection. World J Sci Tec 2011; 1:38-42.
- Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18:268-281.
- Marinho SA, Teixeira AB, Santos OS, et al. Identification of Candida spp. by phenotypic tests and PCR. Braz J Microbiol 2010; 41:286-294.
- Bassetti M, Peghin M, Timsit JF. The current treatment landscape: Candidiasis. J Antimicrob Chemother 2016; 71:13-22.
- Mayer FL, Wilson D, Hube B. Candida albicans pathogenicity mechanisms. Virulence. 2013; 4:119-128.
- Mohammed NA. Detection of Candida spp. and other pathogens responsible for vulvovaginitis in women with contraceptive methods. Doctoral dissertation, MSc thesis. College of Science, University of Baghdad, Iraq 2012.
- Mohammed NA. Molecular detection of biofilm encoding genes in Candida albicans isolated from different sources. Doctoral dissertation, PhD thesis. College of Science, Baghdad University, Iraq 2017.
- Pristov KE, Ghannoum MA. Resistance of Candida to azoles and echinocandins worldwide. Clin Micro Inf 2019; 25:792-798.
- Rigo GV, Tasca T. Vaginitis: Review on drug resistance. Curr Drug Targets 2020; 21:1672-1686.
- Sasani E, Rafat Z, Ashrafi K. Vulvovaginal candidiasis in Iran: A systematic review and meta-analysis on the epidemiology, clinical manifestations, demographic characteristics, risk factors, etiologic agents and laboratory diagnosis. Microb Pathog 2021; 154:104802.
- Sobel JD. Vulvovaginal candidosis. Lancet 2007; 369:1961-1971.
- Sopian IL, Ahmed MA, Lung LT, et al. Yeast infection and diabetes mellitus among pregnant mother in Malaysia. Malays J Med Sci 2016; 23:27.
- Spettel K, Barousch W, Makristathis A, et al. Analysis of antifungal resistance genes in Candida albicans and Candida glabrata using next generation sequencing. PloS one. 2019; 14:e0210397.
- Talapko J, Juzbašić M, Matijević T, et al. Candida albicans-the virulence factors and clinical manifestations of infection. J Fungi 2021; 7:79.
- Tamai IA, Pakbin B, Fasaei BN. Genetic diversity and antifungal susceptibility of Candida albicans isolates from Iranian HIV-infected patients with oral candidiasis. BMC 2021; 14:1-7.
- Wang J, Zhang X, Gao L, et al. The synergistic antifungal activity of resveratrol with azoles against Candida albicans. Lett Appl Microbiol 2021; 72:688-697.
- Wayne RO. Light and video microscopy. Academic Press 2019.
- Yeshiwas D, Mekonnen A. Comparative study of the antioxidant and antibacterial activities of two guava (Psidium guajava) fruit varieties cultivated in Andasa Horticulture Site, Ethiopia. Chem Int 2018; 4:154-162.
- Yuan R, Tu J, Sheng C, et al. Effects of Hsp90 inhibitor ganetespib on inhibition of azole-resistant candida albicans. Front Microbiol 2021; 12:680382.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at , Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Yasser Muwafaq Mehdi*, Rabab Majead Abed and Hadi R. Rasheed Al-Taai
Department of Biology, college of education for pure sciences, Bangalore, IraqCitation: Yasser Muwafaq Mehdi, Rabab Majead Abed, Hadi R. Rasheed Al-Taai, A study of anti-Fungal Resistance Strains of Candida Albicans Yeast Which Isolated from Clinical Specimens of Women Suffering from Vaginal Infections in Baqubah City , J Res Med Dent Sci, 2023, 11(2):36-40.
Received: 09-Jan-2023, Manuscript No. jrmds-22-79653; Accepted: 11-Jan-2023, Pre QC No. jrmds-22-79653; Editor assigned: 11-Jan-2023, Pre QC No. jrmds-22-79653; Reviewed: 25-Jan-2023, QC No. jrmds-22-79653; Revised: 30-Jan-2023, Manuscript No. jrmds-22-79653; Published: 06-Feb-2023
