Case Report - (2022) Volume 10, Issue 11
A Rare Case of Gastritis Due to Iatrogenic Iron Pills in a Child
*Correspondence: Dr. Rajeswaree MB, Department of Paediatrics, Sree Balaji Medical College, Chromepet, Chennai, Tamilnadu, India, Email:
Abstract
Introduction: Iron, a trace mineral in our body that does many functions. Iron is found in red blood cells as haemoglobin and in muscle as myoglobin. The function of Haemoglobin (Hb) is transferring oxygen in blood from lungs to the tissues. Iron pills (supplements), are the treatment of choice for iron deficiency anaemia.
Case report: Here we present a case report of a 10 years old girl who came to the hospital presenting with nausea, upper abdominal pain and poor food intake who is taking iron pills.
Conclusion: If a child presents with gastritis due to iron pills is a rare disease, if left untreated may lead to life threatening complications.
Keywords
Trace mineral, Iron, Haemoglobin, Myoglobin
Introduction
WHO reported the prevalence is around 43% for iron deficiency anaemia worldwide [1]. Iron intake is widely used for the management of iron deficiency anaemia. Absorption of iron occurs in duodenum and proximal jejunum. To be absorbed iron most is in ferrous state Fe2+. Iron deficiency anaemia may lead to many complications, mainly irreversible neurocognitive developmental effects [2].
Ferrous components like ferrous gluconate, ferrous sulphate and ferrous fumarate were absorbed in iron supplements. Iron pills will cause heartburn, constipation and decreases appetite. Ferrous form and ferric form of iron were catalysts that form reactive oxygen metabolite that will cause local damage [3]. But the side effects of gastritis in the paediatric group who consumes iron pills were rare and underreported still.
Case Presentation
A 10 years girl who came to our hospital casualty with complaints of nausea and severe upper abdominal pain and decreased oral intake since 15 days. The girl underwent a kidney transplant 2 years ago due to final stage of renal failure and was on immuno suppressive therapy and was on regular follow up. She took oral iron tablets for treatment of IDA before three months. Prior to endoscopy haemoglobin was 10.9 g/dl, PCV 35%, mean cell volume 108 FL, ferritin 145 μg/L, iron 4, transferrin 30, and transferrin saturation 9%. Endoscopy was done, it showed patchy redness, exudate in the gastric antrum and the first part of the histopathological examination showed sloughed epithelium with lymphocytes and eosinophil’s and few gastric pits were seen. Prussian blue staining was done and there were iron deposits. And patient was diagnosed with iron pill induced gastritis which is iatrogenic. Iron pills were stopped and she was started on omeprazole. Patient improved symptomatically in 2 days, and is on regular follow up and the patient recovered completely.
Discussion
The deposition of iron in the gastric mucosa is called gastric siderosis was described first in 80's [4]. There are three types A,B,C. Type A is accumulation of iron in macrophages, epithelium and stromal. Type B is iron deposition is extracellular and deposited in the vessels. Type C comprises deposition of iron in glandular epithelium of fundic and antral cells. Gastric siderosis is caused by intake of iron pills, blood transfusions, and hemochromatosis. The common cause of gastric siderosis occurs due to oral iron tablets that will lead to gastropathy and termed as Iron pill induced gastritis. One of the Study shows, out of 59 patients with iron deposition, 98% were on iron pills [5]. But the exact pathogenesis is not clearly understood. There are two theories of iron deposition causing gastropathy. First theory is impact of iron tablets leading to side effects that mimics chemical burns. Second theory is uptake with more side effects of iron overload [6].
The prevalence of oral intake of iron pill leading to gastritis is around 0.7% in the adult population [7]. Evaluation of this type of gastritis due to iron pills endoscopy and histopathological examination is required. The clinical features are abdominal pain, discomfort, nausea, malena. But in the paediatric age group it is difficult to gather clinical history.
Endoscopic findings are yellowish or brownish discolouration of mucosa, ulceration, and polyp [8,9]. Gastric siderosis is seen in the mucosa which is either extracellular or intracellular and it involves glands. The pigments are granular, refractile and non-polarizable (Figures 1 and 2).
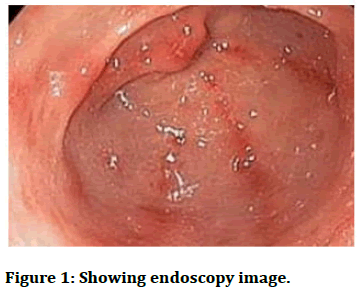
Figure 1: Showing endoscopy image.
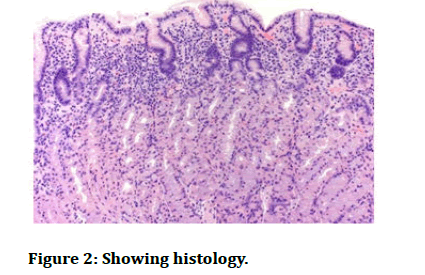
Figure 2: Showing histology.
Ferrous sulphate is associated with gastrointestinal side effects comparatively. In some studies on adult population gastritis due to oral iron pills can be reversed by liquid forms of iron [10-12]. Protein pump inhibitors like Lanzoprazole, Pantoprazole or H2 receptor antagonists like Ranitidine hydrochloride are helpful. Continuous monitoring of side effects is necessary.
Conclusion
Gastritis due to iron pills is common in adult but uncommon in children. So doctors should be aware before prescribing. Parents should be advised on dietary intake and close monitoring of symptoms, warning signs must be explained, so that early management of gastritis is possible.
References
- World Health Organization (WHO). The global prevalence of anaemia in 2011. World Health Organization 2015.
- Ozdemir N. Iron deficiency anaemia from diagnosis to treatment in children. Turk Pediatri Ars 2015; 50:11-19.
- Tenenbein M. Toxic kinetics and toxic dynamics of iron poisoning. Toxicol Lett 1998; 102:653-656.
- Conte D, Velio P, Brunelli L, et al. Stainable iron in gastric and duodenal mucosa of primary hemochromatosis patients and alcoholics. Am J Gastroenterol 1987; 82:237-240.
- Kaye P, Abdulla K, Wood J, et al. Iron induced mucosal pathology of the upper gastrointestinal tract: a common finding in patients on oral iron therapy. Histopathol 2008; 53:311-317.
- Haig A, Driman DK. Iron induced mucosal injury to the upper gastrointestinal tract. Histopathol 2006; 48:808–812.
- Abraham SC, Yardley JH, Wu TT. Erosive injury to the upper gastrointestinal tract in patients receiving iron medication: An under recognized entity. Am J Surg Pathol 1999; 23:1241–1247.
- Levy C, Bongiorno MA, Myint, et al. The endoscopic appearance of focal gastric hemosiderosis as a brown macular patch resembling that of cutaneous iron deposition in hemochromatosis. Am J Gastroenterol 2008; 103:246-248.
- De Petris G, Gatius Caldero S, Chen L, et al. Histopathological changes in the gastrointestinal tract due to drugs: An update for the surgical pathologist (part I of II). Int J Surg Pathol 2014; 22:120–128.
- Marginean EC, Bennick M, Cyczk J, et al. Gastricsiderosis: patterns and significance. Am J Surg Pathol 2006; 30:514-520.
- Kothadia JP, Arju R, Kaminski M, et al. Gastricsiderosis: an under recognized and rare clinical entity. SAGE Open Med 2016; 4.
- Zhang X, Ouyang J, Wieczorek R, et al. Iron medication-induced gastric mucosal injury. Pathol Res Pract 2009; 205:579–581.
Author Info
Department of Paediatrics, Sree Balaji Medical College, Chromepet, Chennai, Tamilnadu, IndiaCitation: Rajeswaree MB, A Rare Case of Gastritis Due to Iatrogenic Iron Pills in a Child, J Res Med Dent Sci, 2022, 10 (11): 156-157.
Received: 02-Sep-2022, Manuscript No. JRMDS-22-57350; , Pre QC No. JRMDS-22-57350(PQ); Editor assigned: 06-Sep-2022, Pre QC No. JRMDS-22-57350(PQ); Reviewed: 21-Sep-2022, QC No. JRMDS-22-57350; Revised: 03-Nov-2022, Manuscript No. JRMDS-22-57350(R); Published: 10-Nov-2022
