Case Report - (2020) Volume 8, Issue 3
A Child with Dihydrolipoamide Dehydrogenase (DLD) Deficiency with a Rare Variant-A Case Report
Ahmed Bin Gaith Al-Mehmadi, Helal Helal Almalki and Aalia Akhtar Hayat*
*Correspondence: Aalia Akhtar Hayat, Department of Pediatrics, Maternity and Children Hospital, Makkah Al Mukarmah, Saudi Arabia, Email:
Abstract
DLD c.685G>T, P. (Gly22gcys) is categorized as pathogenic and has the entrenched role as a disease- causing variant. Diseases caused by DLD variants are inherited in an autosomal recessive fashion. The patient is typically homozygous for the variant. Typically, both parents are carriers of the variant. Each offspring of an affected individual has a 25% probability of being homozygous for the variant, a 50% chance of being an asymptomatic carrier, and a 25% chance of being an unaffected non-carrier. Mutations in DLD are associated with a severe disorder of infancy with failure to thrive, hypotonia, and metabolic acidosis. We presented the case of a 6-year-old child with homozygous splice region missense variant c.685G>T, p. (Gly229Cys) in DLD, her presentation, course of the illness, diagnosis and management in detail. Genetic counseling and family member testing are recommended, and early diagnosis and intervention may prevent the precipitation of an acute episode in such cases.
Keywords
Pyruvate dehydrogenase E3 deficiency, DLD deficiency, E3-deficient maple syrup urine disease, E3 deficiency, Maple syrup urine disease type III, Autosomal recessive disease, Rare gene variant, DLDD, DLDH, E3, GCSL, LAD, PHE3, Genetic diseases, Metabolic disorders, Lipoic acid biosynthesis defects, Pyruvate dehydrogenase complex deficiency
Abbrevations
DLD: Dihydrolipoamide Dehydrogenase, PDH: Pyruvate Dehydrogenase, BCAA: Branched Chain Amino Acids, BCKDH: Branched Chain Alpha- Ketoacid Dehydrogenase, OKGDH: O-Ketoglutarate Dehydrogenase
Introduction
Prevalence of genetic disorders is high in Saudi Arabia accounted by an ominously elevated rate of consanguineous and intertribal marriages along with large family sizes [1]. A significant share of this disease burden is pitched in by autosomal recessive diseases and genetic studies on this populace have not only played an instrumental role in the identification of various novel causal mutations but also in addition of substantial information in the international genetic database [2].
Dihydrolipoamide dehydrogenase (DLD), is also identified as E3 (Dihydrolipoamide: NAD+ oxidoreductase, EC 1.8.1.4), and is a common constituent of more than one mitochondrial complex. It is vital for the process of catalysis. In humans, it is encoded by the DLD gene [3]. Deficiency of DLD as a result of genetic mutations is very rare however if present it is concomitant with a severe disorder of infancy with failure to thrive, hypotonia, and metabolic acidosis. It displays itself with an unlimited degree of unpredictability, ranging from the neurodegenerative and cardiac disease to Friedreich's ataxia and ischemic reperfusion injury [4, 5].
Many variants of DLD have been reported nevertheless we want to account the case of a 6-year-old girl with homozygous splice region missense variant c.685G>T, p. (Gly229Cys) in DLD and to converse her presentation, course of the illness, diagnosis and management in detail.
Case History
The child presented for the first time to our emergency department at the age of 3 years and 10 months (2017) with fever and vomiting. On the physical examination she was oriented but lethargic and slightly jaundiced. The abdomen was slightly tender without ascites. The liver was palpable. Her weight was 13.4 kg whereas height was 93 cm. Her blood pressure was 109/51, temperature was 37.2F and she had tachycardia (100 beats per minute).
Laboratory data at admission demonstrated deranged liver profile, bone profile and prothrombin time. Ultrasonography revealed mild to moderate hepatomegaly (Figure 1) with no other significant finding. A provisional diagnosis of acute liver failure was made, and she was managed conservatively by giving vitamin K, ursodeoxycholic acid, cefotaxime, lactulose, metronidazole and omeprazole. She was moved to inpatient ward till she stabilized and then discharged on recovery after one week.
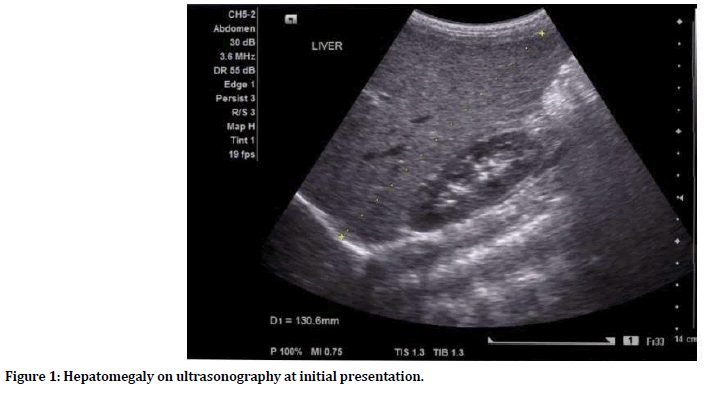
Figure 1. Hepatomegaly on ultrasonography at initial presentation.
She was admitted second time after five months with similar symptoms and was managed along the same lines with additional administration of ondansetron intravenously. She became better and was discharged. However, a subsequent similar episode followed another five months and the additional laboratory tests were performed to exclude all possible causes of liver injury. Hepatitis A, B, C, and E, CMV, and HIV tests turned out to be negative. Autoantibodies were absent; iron studies and ceruloplasmin levels were normal. Abdominal ultrasound revealed hepatomegaly with ascites. Portal and hepatic veins were unremarkable. After ruling out all the possible underlying causes, degenerative liver disease secondary to abnormal genetic variation was considered the most plausible diagnosis.
Family, birth and developmental history
There was a history of strong consanguinity in the family. Two of her sisters were affected; one died at the age of 2 years with status epilepticus followed by stroke while her other sister, (21 years) underwent recurrent admissions with acute liver failure sans any neurological manifestation. Consent to report secondary findings was not received. The perinatal history was unremarkable: she sat at 6 months, walked at 9 months, and controlled sphincters at 14 months. There was no history of social or speech delay. MRI brain was found to be normal.
A sample was sent for genetic testing to look out for any genetic abnormality which failed to yield any positive finding. She was managed along similar lines and discharged after being stable however a sample was taken again and sent for the axon test out of Saudi Arabia. It was not until October 2018 that the result came and confirmed the presence of an underlying abnormal and rare gene variant.
Whole-exome sequence analysis
Whole axon test consists of sequence analysis of all protein coding genes in the genome for the proband. The test targets all protein coding axons, exon intron boundaries and selected noncoding, deep intronic variants. This test is used to detect single nucleotide variants and small insertions and deletions. It may not detect low level mosaicism.
Results
Whole-exome sequence analysis of variants in previously established disease genes test revealed that patient was homozygous for DLD c.685G>T, p(Gly229Cys) which is pathogenic (Table 1). Management plan consisted of supportive therapy at each presentation, nutritional support, and intravenous glucose for hypoglycemia, treatment of metabolic acidosis and correction of coagulopathy. She was booked for regular follow ups to monitor growth and development and plasma amino acid levels (to guide dietary management). Family was counselled and advised to take precautions in order to avoid circumstances precipitating the acute episodes such as fasting, catabolic stressors and liver-toxic medications. She was found to be faring well till her last follow up 6 months ago.
| Gene | Inheritance | Nomenclature | Consequence | Genotype | Phenotype |
|---|---|---|---|---|---|
| DLD | AR (Autosomal recessive) | c.685G>T, p (Gly229Cys) | missense splice_region_variant, gnomAD 77/276964 | HOM (homozygous) | Dihydrolipoyl dehydrogenase deficiency |
Table 1: Variant table: Genetic alterations in established disease genes.
Pyruvate Dehydrogenase (PDH) Complex Deficiency and related deficiencies have a pathological significance [4, 6]. With this report we described a DLD-deficient child with a novel homozygous mutation in the interface domain of DLD. Her predominantly liver failure symptoms and no neurological, developmental or muscular symptoms as opposed to the other variants extend the spectrum of clinical features of this deficiency [7)].
Patients with homozygous variant generally present in early childhood or later as was evident in this case, which is different as compared to typical disease in those who are compound heterozygous and present with very early onset severe disease [6]. The DLD c.685G>T, p.(Gly229cys) variant has been identified in 27 patients with Dihydrolipoamide dehydrogenase (DLD) deficiency, including 22 in a homozygous state like our patient, four in a compound heterozygous state, and one in a heterozygous state in whom a second variant was not identified [8, 9]. Isolated liver disease is found usually in late onset type and may vary between raised liver enzymes alone to fulminant hepatic failure, cirrhosis and death [10]. In this case however, the child presented early with warning signs of acute liver failure and recovered with supportive treatment.
Dihydrolipoamide dehydrogenase functions as the E3 subunit of three mitochondrial enzyme complexes: branched chain alphaketoacid dehydrogenase (BCKDH) complex, o-ketoglutarate dehydrogenase (oKGDH) complex, and pyruvate dehydrogenase (PDH) complex [8]. lt is also a component of the glycine cleavage system and in addition may function as serine protease. The enzyme complexes are an essential component in three major metabolic pathways: the conversion of pyruvate to acetyl-CoA, the Krebs cycle and the branched chain amino acid (BCAA) degradation [11].
In inherited mutations type of DLD deficiency, early-onset typically manifests as a hypotonic infant in contrast to our findings. Affected infants do not survive their initial metabolic decompensation and usually die within the first few years of life. Those who survive, endure both growth and neurodevelopmental abnormalities. Conversely, cases of sole hepatic involvement have a varied age range of presentation that may extend from birth till late thirties [10]. Involvement of numerous metabolic pathways makes management of DLD deficiency challenging. To date, 14 missense and 7 truncating mutations in DLD have been reported in the literature. It has been suggested that the phenotypic heterogeneity in DLD deficiency is not directly correlated with the residual DLD activity but rather with the impact of the mutation on the multi enzymatic complex activity [12].
Recommendations
Screening of family members at risk
If the DLD pathogenic variants in an affected proband are known, at-risk siblings should undergo molecular genetic testing prenatally or after birth so that they can receive appropriate interventions to get management and avoid precipitation of an acute event.
Prenatal and premarital genetic counseling
DLD deficiency is inherited in an autosomal recessive manner. Carrier testing for at-risk relatives and prenatal testing for pregnancies at increased risk are possible if the DLD pathogenic variants in the family are known.
Conclusion
While managing patients with un-explained liver failure symptoms, rare variants of DLD deficiency should be kept in consideration as a possible underlying cause by the clinicians, even if there are no accompanying neurological, developmental or muscular symptoms, particularly in regions like Saudi Arabia where consanguinity is ubiquitous. Early identification using genetic testing in order to make planned choices for prevention is the most promising way to reduce the prevalence of such disorders.
Conflict of Interest
None.
Source of Funding
None.
References
- AbdulAzeez S, Al Qahtani NH, Almandil NB, et al. Genetic disorder prenatal diagnosis and pregnancy termination practices among high consanguinity population, Saudi Arabia. Scientific Reports 2019; 9:17248.
- Monies D, Abouelhoda M, AlSayed M, et al. The landscape of genetic diseases in Saudi Arabia based on the first 1000 diagnostic panels and exomes. Hum Genet 2017; 136:921–939.
- Cameron JM, Levandovskiy V, MacKay N, et al. Novel mutations in dihydrolipoamide dehydrogenase deficiency in two cousins with borderline normal PDH complex activity. Am J Med Genet A. 2006; 140:1542–1552.
- Shaag A, Saada A, Berger I, et al. Molecular basis of lipoamide dehydrogenase deficiency in Ashkenazi Jews. Am J Med Genet 1999; 82:177-182.
- Babady NE, Pang YP, Elpeleg O, et al. Cryptic proteolytic activity of dihydrolipoamide dehydrogenase. Proc Natl Acad Sci USA 2007; 104:6158–6163.
- Patel MS, Harris RA. Mammalian alpha-keto acid dehydrogenase complexes: Gene regulation and genetic defects. J Publ Fed Am Soc Exp Biol 1995; 9:1164–1172.
- Quintana E, Pineda M, Font A, et al. Dihydrolipoamide dehydrogenase (DLD) deficiency in a spanish patient with myopathic presentation due to a new mutation in the interface domain. J Inherit Metab Dis 2010; 33:315-319.
- Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American college of medical genetics and genomics and the association for molecular pathology. Genet Med 2015; 17:405–423.
- Odièvre MH, Chretien D, Munnich A, et al. A novel mutation in the dihydrolipoamide dehydrogenase E3 subunit gene (DLD) resulting in an atypical form of alpha-ketoglutarate dehydrogenase deficiency. Hum Mutat 2005; 25:323–324.
- http://www.ncbi.nlm.nih.gov/books/NBK220444/
- Shany E, Saada A, Landau D, et al. Lipoamide dehydrogenase deficiency due to a novel mutation in the interface domain. Biochem Biophys Res Commun 1999; 262:163–166.
- Stenson PD, Ball EV, Mort M, et al. The human gene mutation database (HGMD) and its exploitation in the fields of personalized genomics and molecular evolution. Curr Protoc Bioinforma 2012; Chapter 1.
Author Info
Ahmed Bin Gaith Al-Mehmadi, Helal Helal Almalki and Aalia Akhtar Hayat*
Department of Pediatrics, Maternity and Children Hospital, Makkah Al Mukarmah, Saudi ArabiaCitation: Ahmed Ghaith Al Mehmadi, Helal Bin Hilal Al Maliki, Aalia Akhtar Hayat, A Child with Dihydrolipoamide Dehydrogenase (DLD) Deficiency with a Rare Variant- A Case Report, J Res Med Dent Sci, 2020, 8 (3):153-156.
Received: 23-Apr-2020 Accepted: 18-May-2020
